Gold in the oxidation state +III is useful, for example, in catalysis, in photoluminescent materials, and in anti-cancer agents. However, fundamental aspects of the structure, bonding, and chemical reactivity of Au(III) compounds are poorly understood, in stark contrast to platinum, its neighbor in the periodic table. One aspect that could be important, e.g., for the medicinal action of gold, is the ability to form hydrogen bonds. H-bonding is well known for Pt(II) compounds.
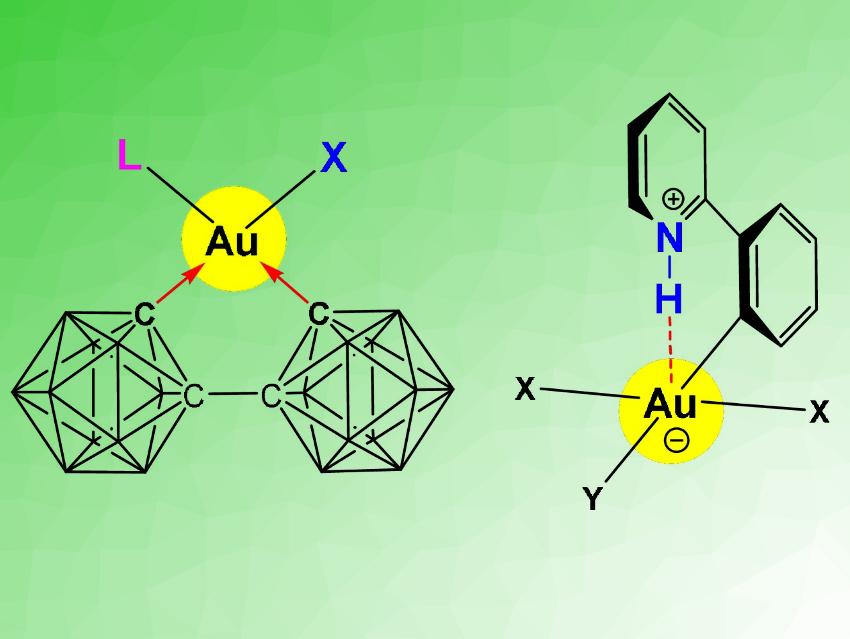
Manfred Bochmann, University of East Anglia, Norwich, UK, Peter Budzelaar, University of Naples, Italy, and colleagues have used a combined synthetic and computational approach to study previously unknown dicarboranyl complexes of gold(III) (general structure pictured left). This type of complex was prepared via a reaction of Li2[(2,2’‐C2B10H10)2] with a cyclometallated gold(III) 2‐phenylpyridine dichloro complex. The reactivity in such complexes is strongly determined by the trans-influence of the ligands. Dicarboranyls are electron-withdrawing. This reduces the rate of ligand exchange and stabilizes the complexes.
The team then investigated whether Au⋅⋅⋅H–N hydrogen bonds involving the pyridine groups (pictured right) make an energetically meaningful bonding contribution. The results of density functional theory (DFT) calculations showed the absence of such interactions for gold(III) in this type of complex. This is a surprising difference in bonding between isoelectronic Au(III) and Pt(II) systems.
- Do Gold(III) Complexes Form Hydrogen Bonds? An Exploration of AuIII Dicarboranyl Chemistry,
Isabelle Chambrier, David L. Hughes, Rebekah J. Jeans, Alan J. Welch, Peter H. M. Budzelaar, Manfred Bochmann,
Chem. Eur. J. 2020.
https://doi.org/10.1002/chem.201904790