The Haber process for producing NH3 is the most important chemical process developed in the 20th century. At 400 to 500 °C and 150 to 200 bar, N2 and H2 react to form NH3. The required H2 is produced by the reformation of CH4 or coal with H2O. For each ton of NH3 produced, approximately 2 t CO2 are emitted. The elimination of this CO2 emission as well as to omit the use of carbon sources for H2 production are highly desired. Around 80 % of the produced NH3 is converted to be used as fertilizer.
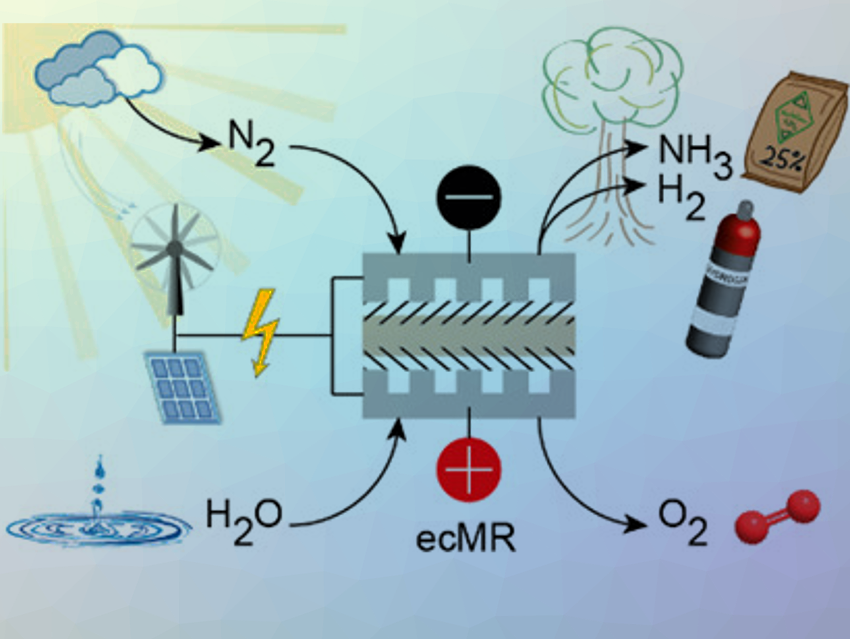
A potentially more sustainable NH3 synthesis method is the direct reduction of N2 using an electrochemical membrane reactor (ecMR). It avoids CO2 emission, if renewable energy sources, such as wind or solar power, are used to power the process.
An ecMR consists of an anode for water oxidation, a proton exchange membranes (PEM), and a cathode for the desired reduction as well as parallel reactions. The workability of the ecMR for the electrochemical reduction of CO2 to synthesize hydrocarbons was proved, however, at very low production rates and current efficiencies.
Matthias Wessling and colleagues, DWI – Leibnitz Institute for Interactive Materials, Aachen, Germany, have used an ecMR process for the simultaneous generation of NH3 and H2 (pictured). The process is carbon‐independent and CO2‐free: H2O is used as an abundant source of H2, and renewable energy can potentially drive the reaction.
The core of the ecMR is the membrane electrode assembly (MEA). It combines (i) the oxygen evolution reaction (OER) at the anode (H2O is oxidized to form O2 and electrons), (ii) the migration of H+ ions across the polymer cation exchange membrane (CEM) to the cathode, and (iii) the reduction of N2 to NH3 at the cathode. The main (parallel) reaction at the cathode is a reduction of H+, i.e., the hydrogen evolution reaction (HER). The MEA presented here consisted of three elements: a) an iridium mixed metal oxide (IrMMO) anode, b) a polymer CEM, and c) a Ti cathodic catalyst
According to the researchers, the ecMR demonstrates the workability to co‐generate NH3 and H2 directly from nitrogen and water vapor at ambient conditions. An exceptional high NH3‐specific current efficiency of up to 27.5% is reported. The co‐generation can be tuned by the balance of process parameters. This synthesis is a viable option to store energy and produce fertilizer precursors.
According to the researchers, their work encourages further research on catalysts for the electrochemical synthesis of NH3 to make the process more efficient in terms of NH3 selectivity and production rates. In their opinion, there are several ways to improve the achieved production rate and current efficiency of the ecMR.
- Co‐generation of Ammonia and H2 from H2O Vapor and N2 Using a Membrane Electrode Assembly,
Kurt Kugler, Stefanie M. A. Kriescher, Martin Giela, Schwan Hosseiny, Kristof Thimm, Matthias Wessling,
Chem. Ing. Tech. 2020.
https://doi.org/10.1002/cite.201900090