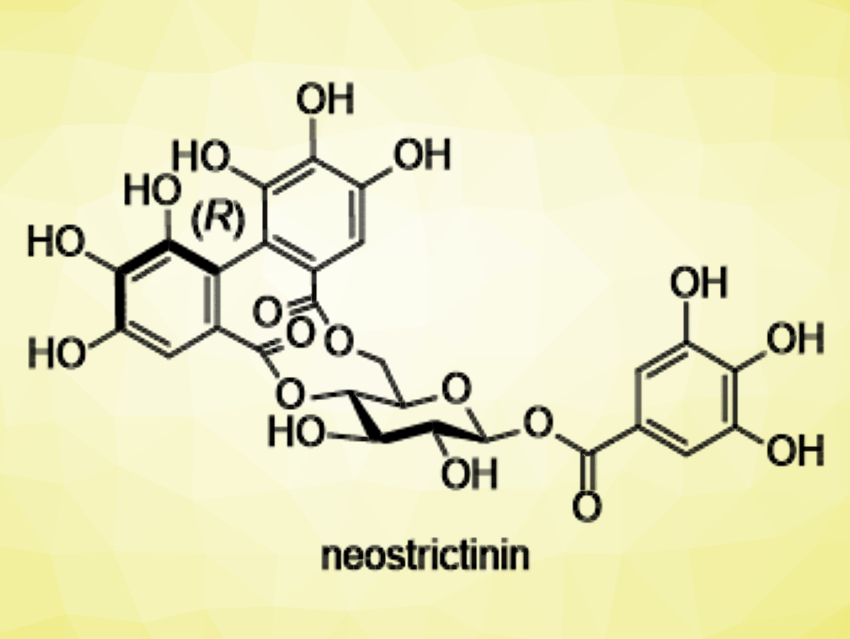
Neostrictinin (pictured) is an ellagitannin, a type of tannin. It features an (R)-hexahydroxydiphenoyl (HHDP) bridge (pictured in red) between the 4- and 6-oxygens of D-glucose. Although more than 300 species of 4,6-O-HHDP bridged ellagitannins have been isolated to date, only four compounds, including neostrictinin, have (R)-axial chirality in the HHDP bridge. This indicates that the (R)-configured bridge is less stable than the (S)-configured bridge. There had been no synthetic methods to access the 4,6-O-(R)-HHDP bridge so far.
Kazutada Ikeuchi, Hidetoshi Yamada, Kwansei Gakuin University, Sanda, Japan, and colleagues have synthesized this labile bridge via a two-step bislactonization strategy (pictured below). One important step to the successful preparation is the excess use of a Mukaiyama condensation reagent (2-chloro-N-methylpyridinium iodide) in the intramolecular lactonization of a seco acid with an (R)-HHDP moiety at the 6-oxygen of the sugar intermediate. The reaction proceeds via the dimerization of the seco acid to provide the 4,6-O-(R)-HHDP bridged compound in 29 % isolated yield.
Based on this key transformation, the team achieved the first total synthesis of neostrictinin in 13 % overall yield. The team also obtained epi-neostrictinin in a yield of 8.7 %.
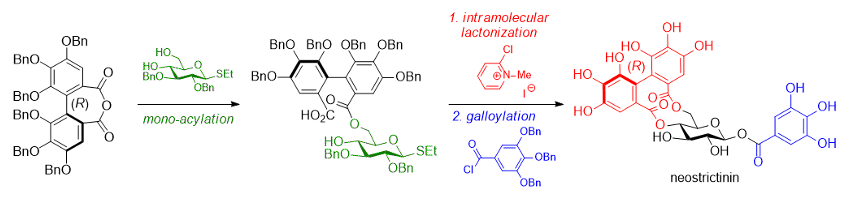
- First Total Synthesis of Neostrictinin,
Kazutada Ikeuchi, Tatsuya Ueji, Shinnosuke Matsumoto, Shinnosuke Wakamori, Hidetoshi Yamada,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000053