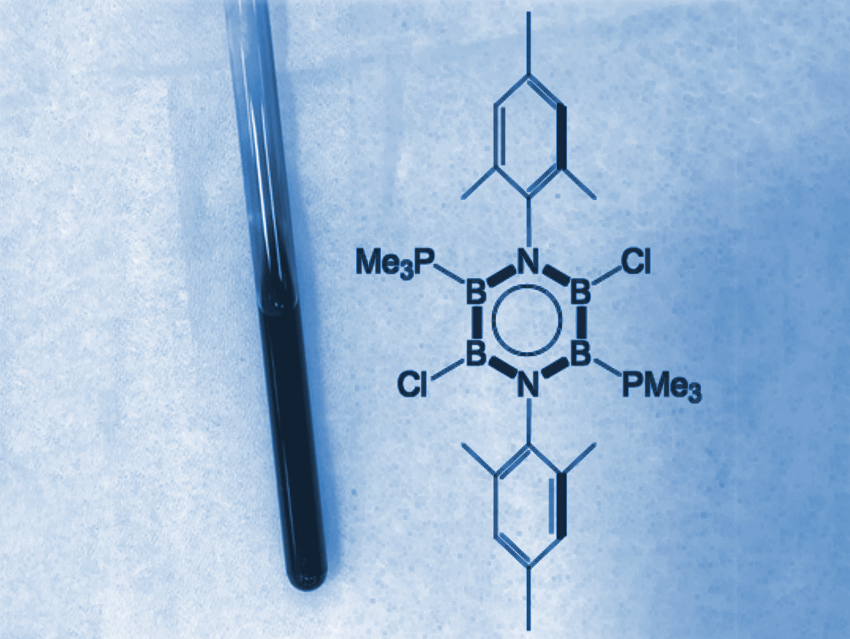
Benzene (C6H6) is an aromatic molecule. The aromaticity of “inorganic benzene”, i.e., borazine (B3N3H6), remains controversial, mainly due to the significantly polarized B–N bonds. Thus, the synthesis of aromatic inorganic benzene derivatives is still a challenge. Verified aromatic character is limited to a few heavier analogues.
Kei Ota and Rei Kinjo, Nanyang Technological University, Singapore, have synthesized a neutral, boron-rich inorganic benzene analogue (pictured). The team first synthesized a cyclic diazatetraborinane (B4N2) precursor via the deprotonation of a bis(anilide)bis(dimethylamido)diborane with n‐butyllithium, followed by treatment with bis(dimethylamido)diborondichloride. The resulting cyclic intermediate was chlorinated using boron trichloride (BCl3). Finally, the researchers reduced the chlorinated precursor with potassium graphite in the presence of trimethyl phosphine (PMe3). They obtained deep blue crystals of the desired 1,4,2,3,5,6-diazatetraborinine derivative.
This product was characterized by NMR spectroscopy, UV/Vis spectroscopy, and X-ray diffraction. The bonding situation was investigated using density functional theory (DFT) calculations. The structural and electronic properties of the molecule confirm that the planar central B4N2 ring has a pronounced aromatic nature, similar to benzene. According to the researchers, this result could aid the design of new aromatic materials.
- A Neutral and Aromatic Boron-Rich Inorganic Benzene,
Kei Ota, Rei Kinjo,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201915790