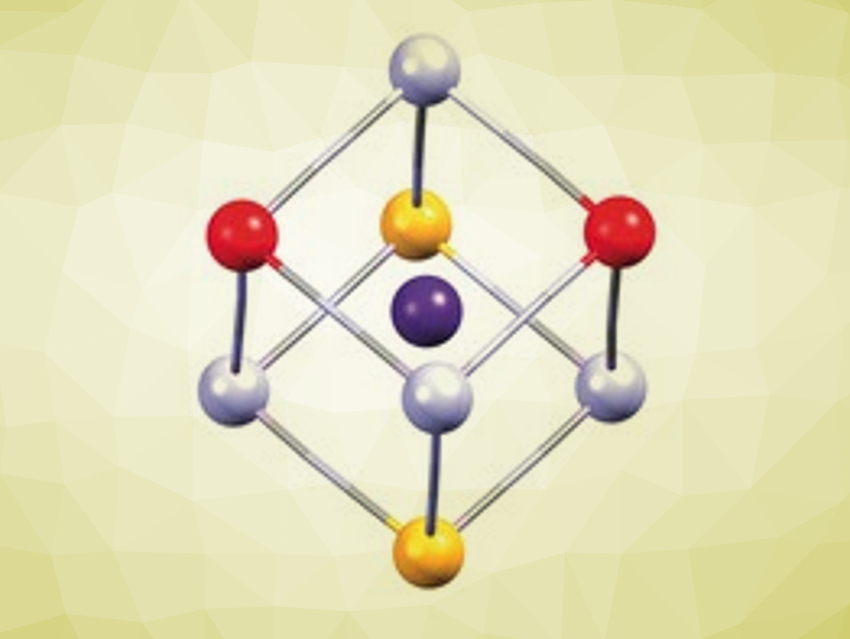
Controlling the magnetic or optical properties of a molecule through an external stimulus is an interesting but challenging task. Such switchable properties are known in cyanide-based inorganic polymers such as RbMn[Fe(CN)6]. So far, however, they have not been reproduced in discrete FeMn complexes.
Rodrigue Lescouëzec, Institut Parisien de Chimie Moléculaire, France, and colleagues have synthesized a model complex of the FeMn-based inorganic polymer. By assembling Fe and Mn complexes that are partially blocked with tridentate ligands, a cubic Fe4Mn4 complex (pictured) can be formed in solution. The cube formation is favored in concentrated solution and in the presence of a templating Cs+ ion that stabilizes the framework from within.
The compound was prepared by a reaction between PPh4[FeIII(Tp)(CN)3] (Tp = hydrotris(pyrazol‐1‐yl)borate), MnII(ClO4)2⋅6 H2O, and Na[pzTp] (pzTp = tetrakis(pyrazol‐1‐yl)borate) in the presence of an excess of cesium iodide in acetonitrile. The product was obtained as red prismatic single crystals by slow evaporation.
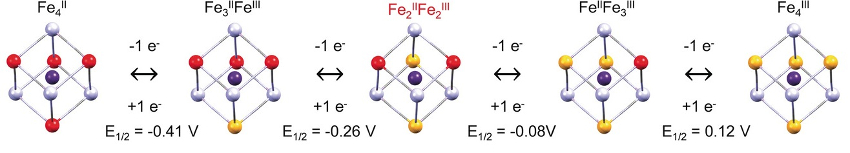
Magnetic and Mössbauer measurements show an FeII-MnIII to FeIII-MnII conversion can be induced by a temperature increase. The Fe4Mn4 cube, which is stable in solution, can be reversibly switched between five different electronic states (pictured below), leading to interesting electrochromic properties. The team observed this by recording cyclic voltammograms and UV/Vis absorption spectra. According to the researchers, they will study other MnFe model complexes with different ligands and intercalated cations to provide a better understanding of this phenomenon.
- Electron Transfer in the Cs⊂{Mn4Fe4} Cubic Switch: a Soluble Molecular Model of the MnFe Prussian-Blue Analogues,
Juan-Ramón Jiménez, Jana Glatz, Amina Benchohra, Geoffrey Gontard, Lise-Marie Chamoreau, Jean-François Meunier, Azzedine Bousseksou, Rodrigue Lescouëzec,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.201916199