Alkaline earth metals could be useful for homogeneous catalysis due to their high natural abundance. In general, their performance increases when going down the group from Mg to Ba. As such, barium catalysis is most attractive. However, alkaline earth metal complexes can be subject to Schlenk equilibria, which lead to a redistribution of ligands and, thus, to ill-defined species. This problem becomes more pronounced as the size of the metal increases, which is why many researchers focus on Mg and Ca.
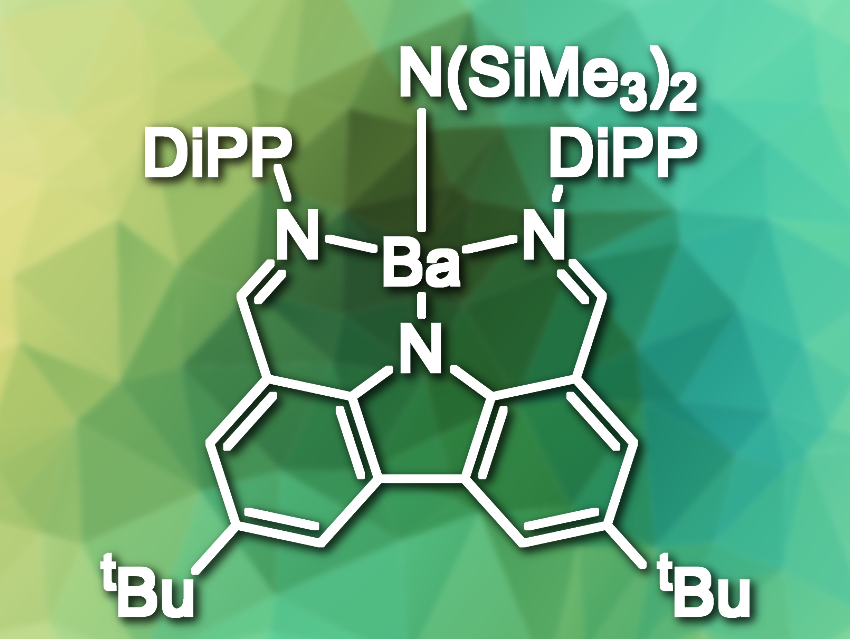
Yann Sarazin and colleagues, University of Rennes, France, have found that a bisiminocarbazolate ligand can suppress this Schlenk redistribution. The team synthesized several previously unknown barium complexes using this ligand, e.g., a barium fluoride and two barium tetrelide complexes (tetrel = Si,Sn). The researchers also prepared a barium amide complex (pictured, DiPP = 2,6-diisopropylphenyl) that acts as a catalyst for the formation of C–P bonds via the hydrophosphination of styrene and phosphines.
The ligand was synthesized by a condensation between 2,6-diisopropylaniline and 1,8-diformyl-3,6-di-tert-butylcarbazole. The barium amide complex was then prepared by a reaction with [Ba{N(SiMe3)2}2]2. The amide can react with Me3SnF to give a fluoride complex. The tetrelide complexes were prepared via a reaction of the ligand with potassium hydride, followed by BaI2 to give a barium complex with an iodide ligand and finally, tetrahydrofuran (THF) adducts of KSi(SiMe3)3 or KSn(SiMe3)3 to give the desired product.
According to the team, the bisiminocarbazolate ligand could allow the synthesis of other unprecedented barium compounds. In addition, its coordination chemistry with other metals could be worth investigating.
- Bis(imino)carbazolate: a master key for barium chemistry,
Peter Chapple, Samia Kahlal, Julien Cartron, Thierry Roisnel, Vincent Dorcet, Marie Cordier, Jean-Yves Saillard, Jean-Francois Carpentier, Yann Sarazin,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202001439