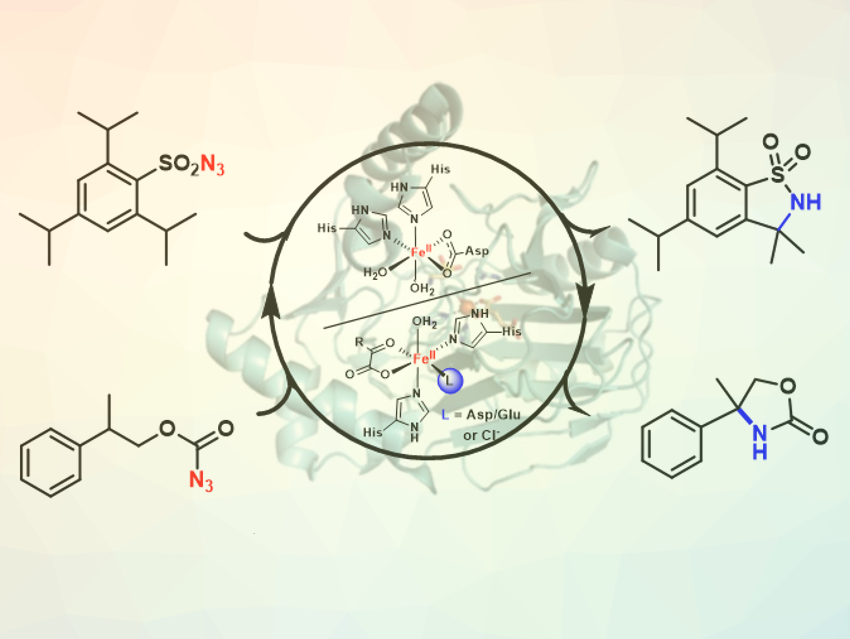
Direct C–H aminations via nitrene transfer provide a useful synthetic strategy for the preparation of amines. These reactions are traditionally performed using transition-metal catalysts. However, “nitrene transferase” enzymes have recently emerged as an alternative to promote these transformations via mild, cost-effective, and environmentally friendly biocatalytic processes.
Ignacio Carrera, Universidad de la República, Montevideo, Uruguay, Rudi Fasan, University of Rochester, NY, USA, and colleagues have tested a range of functionally and structurally different non‐heme iron‐dependent oxidases for their C−H amination activity. They found that several of these enzymes, including Rieske dioxygenases and α‐ketoglutarate‐dependent dioxygenases and halogenases, can function as C–H aminases via nitrene transfer chemistry.
The most promising catalyst, naphthalene dioxygenase (NDO), showed high conversion and chemoselectivity in a gram-scale biotransformation of a sulfonyl azide substrate into the corresponding C-H amination product. This enzyme is reasonably oxygen tolerant, in contrast to previously reported hemoprotein-based biocatalysts, which require strictly anaerobic conditions. This work could be a basis for further studies of transformations using non-heme iron-dependent enzymes.
- C-H Amination via Nitrene Transfer Catalyzed by Mononuclear Non-Heme Iron-Dependent Enzymes,
Maria Agustina Vila, Viktoria Steck, Sonia Rodriguez Giordano, Ignacio Carrera, Rudi Fasan,
ChemBioChem 2020.
https://doi.org/10.1002/cbic.201900783