Atomic layer deposition (ALD) is a chemical vapor deposition (CVD) technique that allows the creation of thin films with high uniformity. ALD does not require harsh conditions but is fairly demanding in terms of suitable precursors.
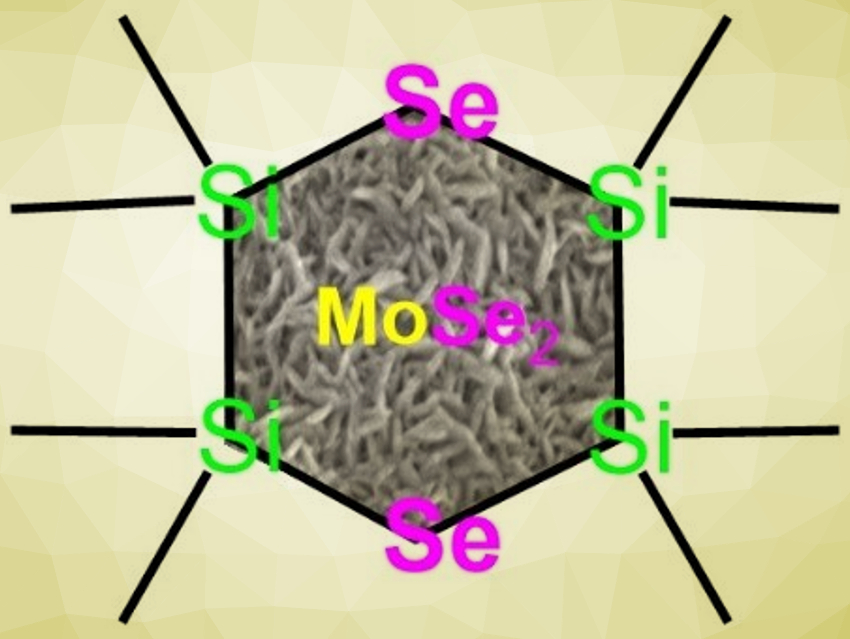
Transition metal dichalcogenides (TMDs), such as diselenides, have properties that are useful in, e.g., photocatalysis, hydrogen evolution, or semiconductor applications. Very thin and uniform TMD layers fabricated by ALD are highly desirable in terms of improving the material’s technological performance. Unfortunately, the range of ALD precursors that act as a selenium source is very limited. H2Se, for example, is highly toxic, while Et2Se and Et2Se2 allow less control over the deposition process. Linear bis(trialkylsilyl)selenides (R3Si−Se−SiR3) are an alternative, but these compounds are prone to hydrolysis and oxidation, and thus, hard to handle.
Filip Bureš, University of Pardubice, Czech Republic, and colleagues have extended the portfolio of Se precursors with three cyclic silyl-selenides. These compounds possess properties similar to bis(trialkylsilyl)selenides but have improved resistance towards moisture and air, which allows for easier handling. The team first synthesized lithium selenide via a reaction of elemental selenium with lithium triethylborohydride. The resulting Li2Se was treated with either 1,2‐bis(chlorodimethylsilyl)ethane, 1,3‐bis(chlorodimethylsilyl)propane, or two equivalents of 1,2‐dichlorotetramethyldisilane to give five- or six-membered cyclic Se precursors (pictured below).
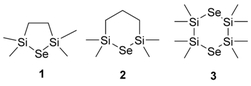
All three prepared cyclic silyl-selenides react with MoCl5 to give MoSe2 nanosheets under ALD conditions. The six-membered cyclic selenide containing two selenium atoms (3, pictured) proved to be the best Se precursor in the group.
- Cyclic Silylselenides: Convenient Selenium Precursors for Atomic Layer Deposition,
Jaroslav Charvot, Daniel Pokorný, Raul Zazpe, Richard Krumpolec, David Pavliňák, Luděk Hromádko, Jan Přikryl, Jhonatan Rodriguez‐Pereira, Milan Klikar, Veronika Jelínková, Jan M. Macak, Filip Bureš
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000108