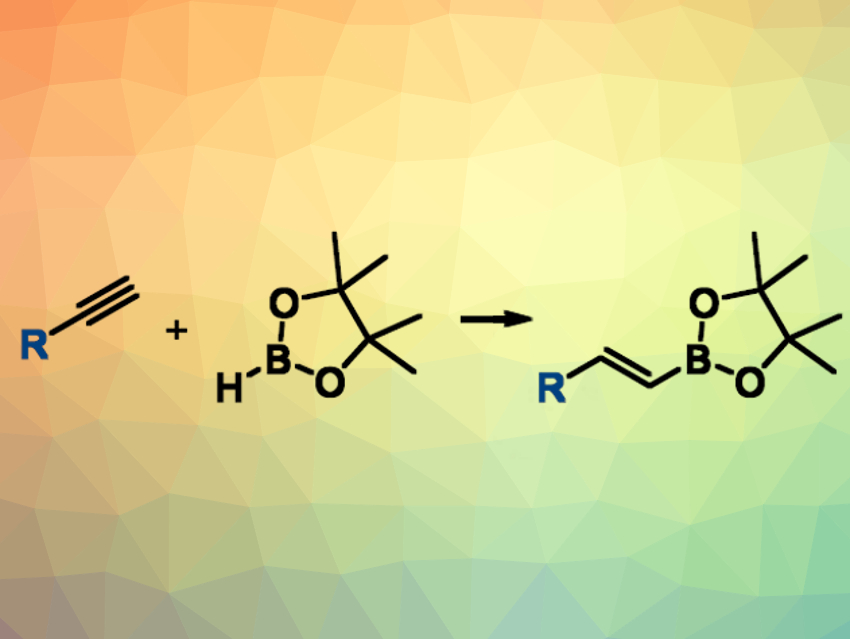
Alkenyl boronic esters are versatile intermediates in organic synthesis. This is due to the wide range of options to further transform C–B bonds into other functional groups. Alkenyl boronic esters can be synthesized. e.g., by the hydroboration of alkynes. Microwave irradiation, in combination with different catalysts, can be used to improve the efficiency of this transformation.
Javier Carreras and colleagues, Universidad de Alcalá, Madrid, Spain, have developed a simple and fast microwave-assisted hydroboration of terminal alkynes with pinacolborane under air that does not require the use of any additive or solvent. The reactants were simply heated under microwave irradiation. This reaction (pictured) gives the desired (E)-alkenyl boronates in good yields and with short reactions times (20 min) at 215 °C. No catalyst was added, but the researchers caution that they cannot exclude the presence of catalytically active palladium traces in the alkynes or catalytically active decomposition products of pinacolborane.
The protocol shows good functional group tolerance. The researchers were able to perform further transformations at the C–B bond without purification of the alkenyl boronate intermediate. This two-step process provides access to vinyl iodines, vinyl azides, vinyl ethers, stilbene derivatives, or trifluoroborate salts.
- Practical solvent-free microwave-assisted hydroboration of alkynes,
Julia Altarejos, David Sucunza, Juan J. Vaquero, Javier Carreras,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000110