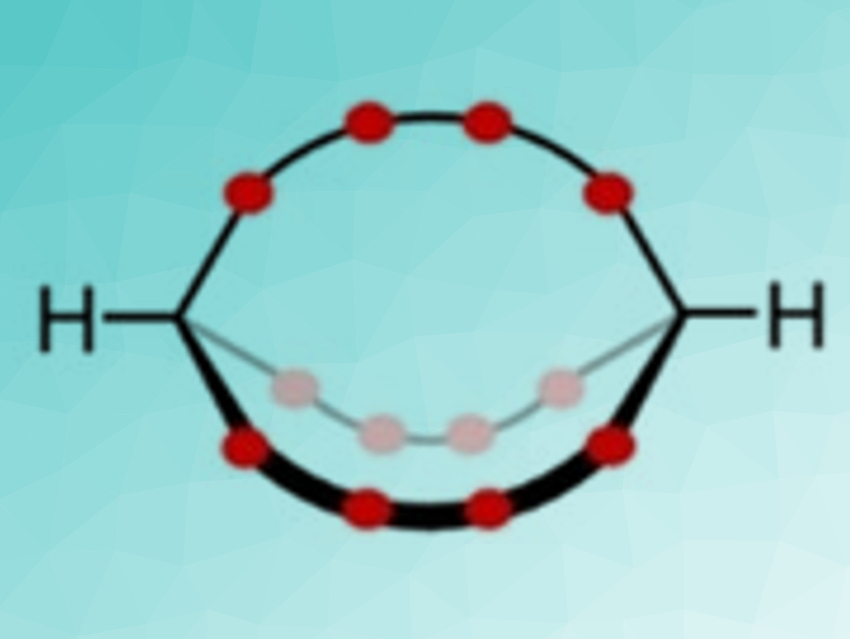
Certain cryptands (example pictured above) show a special type of stereoisomerism, in which substituents or lone pairs at the bridgehead atoms can point either into the cryptand’s cavity or away from it. Such large macrocycles can invert between these out,out- and in,in-isomers via a so-called homeomorphic isomerization. In this process, both bridgeheads invert by pulling one chain through the ring defined by the other two—without any bonds breaking.
Christof M. Jäger, University of Nottingham, UK, Max von Delius, University of Ulm, Germany, have investigated dynamic orthoester-based cryptands. They studied the homeomorphic isomerization between out,out– and in,in-2.2.2-cryptands (schematically pictured below, oxygen atoms in red). The cryptands were synthesized from trimethoxy orthoformate and triethylene glycol (TEG), using large cesium cations as a template.
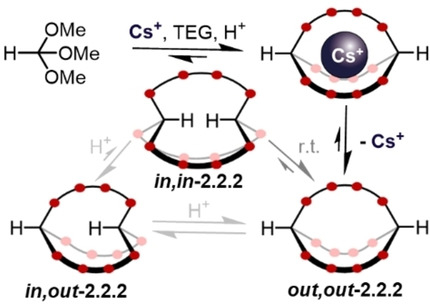
The team found that a third in,out-stereoisomer was formed when trifluoroacetic acid (TFA) was added to induce dynamic exchange of the orthoformate. The system could be driven to this in,out-state by using a smaller sodium metal template. The size of Na+ is a mismatch for the cavity of the out,out-stereoisomer, and thus, using sodium ions favors the formation of the in,out-cryptand.
In addition to experiments, the team used density functional theory (DFT) calculations and molecular dynamics (MD) simulations to better understand the systems. The animated gif below, for example, shows the homeomorphic inversion of an orthoester out,out-cryptand to its in,in-isomer via a representative pathway based on MD simulations.
- Self‐Assembly, Adaptive Response, and in,out‐Stereoisomerism of Large Orthoformate Cryptands,
Henrik Löw, Elena Mena‐Osteritz, Kathleen M. Mullen, Christof M. Jäger, Max Delius,
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000254