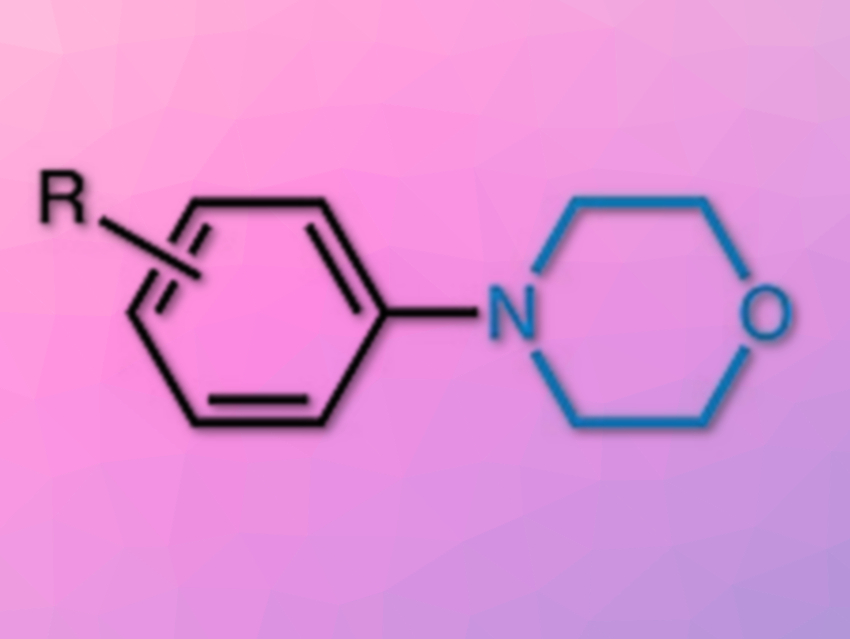
Substituted anilines are widely found in pharmaceuticals, bioactive natural products, and advanced materials. One approach to their synthesis is the copper‐promoted oxidative amination of arylboronic acids, i.e., the Chan–Lam coupling. Nucleophiles for Chan–Lam‐type aminations usually are arylmetal species or arylsilanes.
Jun Shimokawa, Hideki Yorimitsu and colleagues, Kyoto University, Japan, have developed new conditions for the synthesis of substituted anilines from arylsilanes (example pictured below). Unlike the Chan-Lam conditions, in which a stoichiometric amount of copper salt is required, this reaction uses a catalytic amount of an NHC-copper complex (pictured below). It allows simple di- or trialkoxysilyl groups to be converted to amino groups. O-benzoylhydroxylamines act simultaneously as the amine sources and reoxidizing agents, and AgF is used as a base and as an activator for the silyl groups. The reaction is performed in toluene at 50 °C.
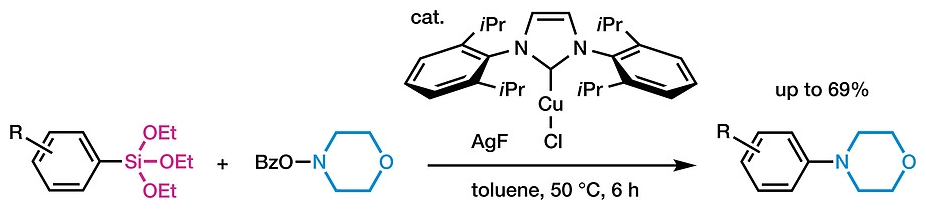
The desired products were obtained in generally moderate yields. Triethoxyarylsilanes with electron-withdrawing or -donating groups on the aromatic rings could be transformed into the desired tertiary aniline derivatives. The team found that AgF is indispensable as an activator; other fluoride salts such as CsF do not mediate the reaction.
- Copper-catalyzed Electrophilic Amination of Alkoxyarylsilanes,
Qian Zhang, Kenshiro Hitoshio, Hayate Saito, Jun Shimokawa, Hideki Yorimitsu,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000562