Iron phosphide nanoparticles show promise as electrocatalysts, for example, for the hydrogen evolution reaction (HER). One way to synthesize iron phosphide nanomaterials is to insert phosphorus into well‐defined iron nanoparticles. However, there is a lack of reactive, yet safe, phosphorus sources for the selective formation of crystalline compounds.
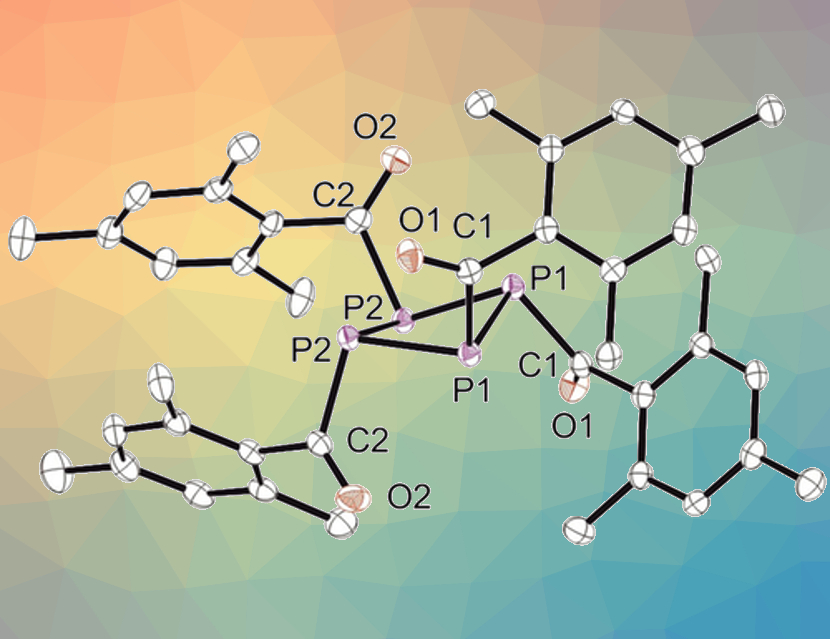
Sophie Carenco, Sorbonne Université, Paris, France, and colleagues have developed a synthetic route to form tetrakis(mesitoyl)cyclotetraphosphane, P4(MesCO)4 (crystal structure pictured above), which can be used as a P source. The team first prepared mesitoyl phosphane (MesCO–PH2) by reacting [NaPH2 × Na(OtBu)x] with mesitoylmethylester. The mesitoyl phosphane was then reacted with hexachloroethane and trimethylamine to give the desired product in an isolated yield of 10 %.
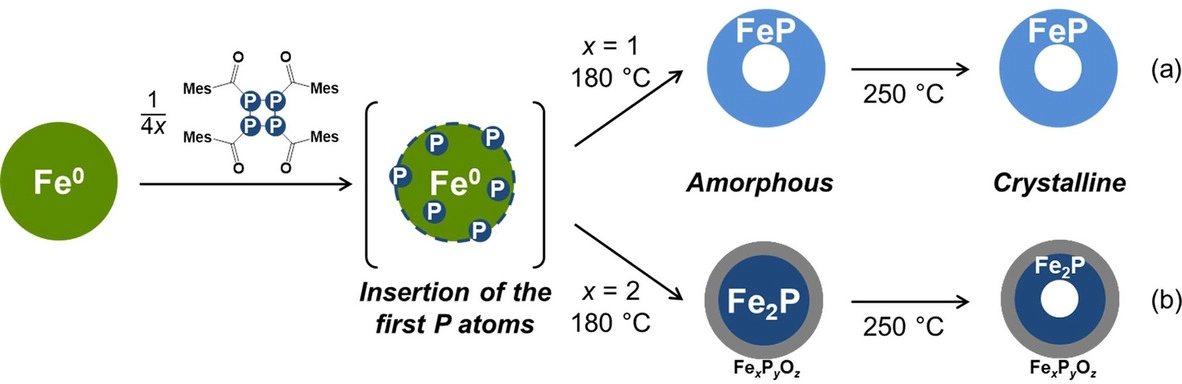
The researchers reacted P4(MesCO)4 with colloidal iron nanoparticles to form FeP and Fe2P nanoparticles of similar diameters (pictured below). They obtained hollow, crystalline FeP and Fe2P nanoparticles when the reaction was performed at 250 °C. At a lower temperature of 180 °C, amorphous nanoparticles were obtained. These phases were also formed as an intermediate in the synthesis of the crystalline particles.
The team investigated the electrocatalytic properties of amorphous and crystalline FeP nanoparticles, as well as crystalline Fe2P nanoparticles, for the hydrogen evolution reaction (HER) in acidic and neutral electrolytes. In both electrolytes, amorphous FeP was a more efficient catalyst than crystalline FeP, and crystalline Fe2P was the least active catalyst. According to the researchers, these results could provide a path to a more systematic investigation of amorphous metal phosphide phases in electrocatalysis.
- A Single Molecular Stoichiometric P‐Source for Phase‐Selective Synthesis of Crystalline and Amorphous Iron Phosphide Nanocatalysts,
Florian D’Accriscio, Erik Schrader, Capucine Sassoye, Mohamed Selmane, Rémi F. André, Sarah Lamaison, David Wakerley, Marc Fontecave, Victor Mougel, Grégoire Le Corre, Hansjörg Grützmacher, Clément Sanchez, Sophie Carenco,
ChemNanoMat 2020.
https://doi.org/10.1002/cnma.202000198