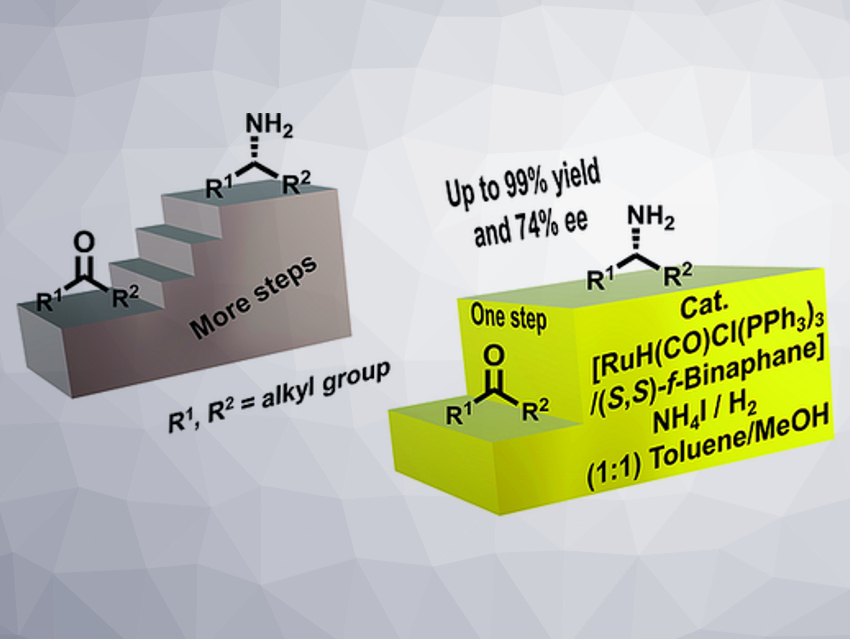
Primary chiral aliphatic amines are important building blocks, e.g., for the synthesis of pharmaceuticals and agrochemicals. Most state-of-the-art syntheses for this class of molecules involve multi-step procedures, require protecting groups, or use enzymes that are very substrate-specific. The use of selective, chiral metal catalysts and simple ammonia sources in combination with hydrogen as the reductant for the asymmetric amination of aliphatic ketones could be an elegant alternative to these methods. However, existing catalyst systems provide very low enantioselectivities (less than 30 %ee) for these substrates.
Thomas Schaub, Catalysis Research Laboratory (CaRLa), Heidelberg, Germany, and BASF SE, Ludwigshafen, Germany, and colleagues have optimized this type of amination. The team used [Ru(PPh3)3H(CO)Cl] as a catalyst, together with (S,S)-f-binaphane as a chiral ligand, NH4I as the ammonia source, and a mixture of toluene and methanol as the solvent to convert a variety of aliphatic ketones to the desired amines under a hydrogen atmosphere.
The team achieved unprecedented enantioselectivities of up to 74% ee for these substrates. This work shows that it is possible to obtain significant enantioselectivities in the direct reductive amination of aliphatic ketones with metal catalysts. This could provide a starting point for the further development and improvement of this reaction.
- Ruthenium Catalyzed Direct Asymmetric Reductive Amination of Simple Aliphatic Ketones Using Ammonium Iodide and Hydrogen,
Tamal Ghosh, Martin Ernst, A. Stephen K. Hashmi, Thomas Schaub,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000750