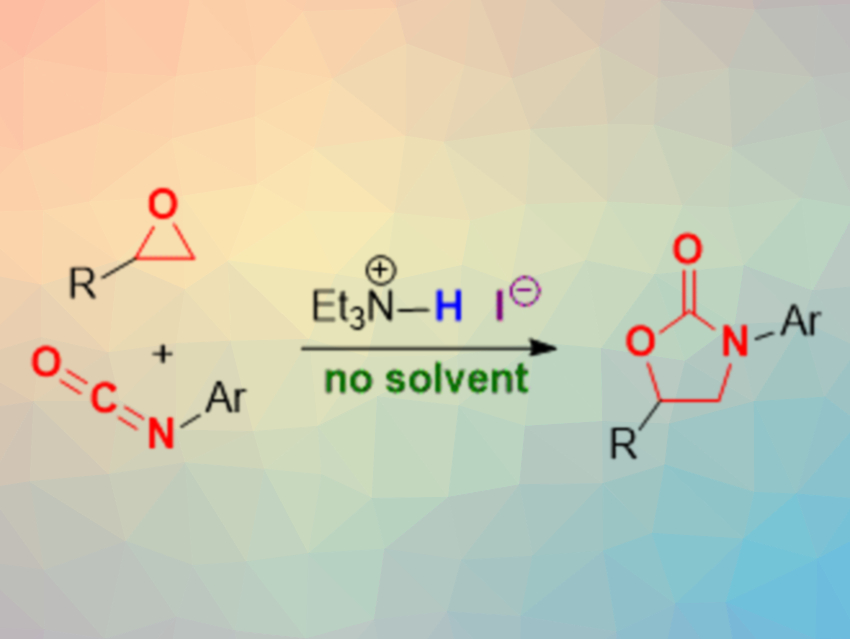
Among the wide variety of five-membered heterocycles, 2-oxazolidinones are particularly important, e.g., in medicinal chemistry. Many important pharmaceutical agents contain a 2-oxazolidinone core. Therefore, practical methods for the synthesis of these heterocycles are useful.
Seiji Shirakawa and colleagues, Nagasaki University, Japan, have developed a method for the synthesis of 2-oxazolidinones under solvent- and metal-free conditions, using triethylamine hydroiodide as a simple and effective bifunctional organocatalyst (pictured above). The team reacted different epoxides and isocyanates using triethylamine hydroiodide as the catalyst without a solvent at 100 °C. The desired 2-oxazolidinones were obtained in good to excellent yields.
The triethylamine hydroiodide catalyst combines a Brønsted-acidic hydrogen with a nucleophilic iodide ion. This dual functionality allows the activation of the epoxide via a hydrogen-bond interaction, followed by a nucleophilic attack of the iodide at the three-ring to give an alkoxide intermediate. This nucleophilic intermediate can attack the isocyanate, followed by a ring-closing to give the product and release the iodide. According to the researchers, this reaction is one of the most economical processes documented for the synthesis of 2-oxazolidinones so far.
- Triethylamine Hydroiodide as a Bifunctional Catalyst for the Solvent-Free Synthesis of 2-Oxazolidinones,
Ryuichi Nishiyori, Ken Okuno, Seiji Shirakawa,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000771