Metal–olefin complexes are often used in synthetic organic chemistry. Olefins with planar chirality, in particular, have contributed significantly to advancements in asymmetric transition-metal catalysis. Trans-cyclooctene, which was the first chiral olefin synthesized, can coordinate to transition metals and binds strongly due to its strained structure. However, the behavior of trans-cyclooctene derivatives as chiral ligands for asymmetric catalysis had been unknown so far.
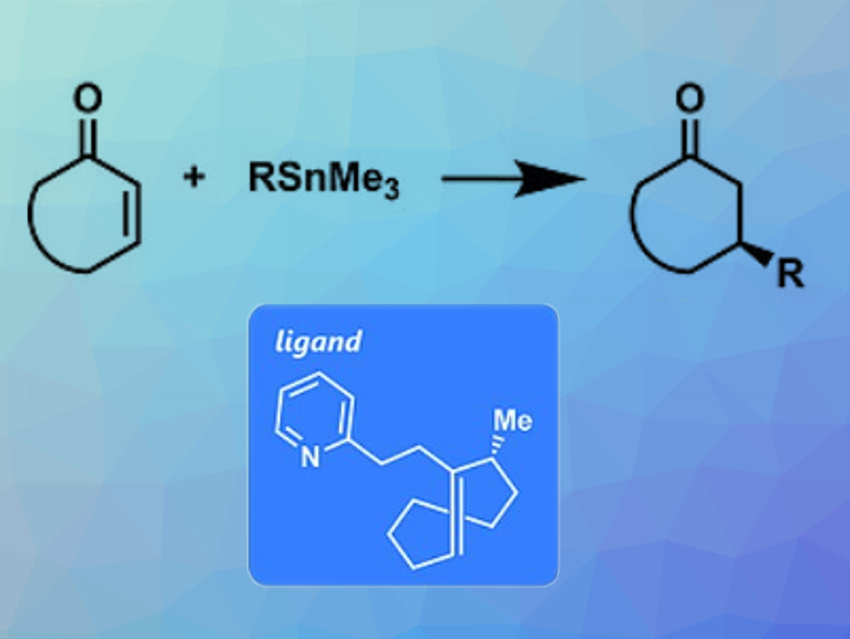
Keisuke Asano, Seijiro Matsubara, and colleagues, Kyoto University, Japan, have used trans-cyclooctenes as asymmetric ligands for rhodium-catalyzed 1,4-additions of organotin reagents (pictured). The team synthesized chiral ligands (example pictured in the blue box) starting from cyclooctanone via an epoxidation, a ring-opening with (pyridin-2-ylmethyl)lithium, and a conversion to the corresponding cis‐cyclooctene. This intermediate was converted to the racemic trans‐cyclooctene via another epoxidation, a ring-opening with lithium diphenylphosphide, oxidation to the phosphine oxide, and treatment with sodium hydride. The enantiomers were subsequently separated by liquid column chromatography with a chiral stationary phase.
The researchers then used the ligands together with [RhCl(C2H4)2]2 as catalyst systems for the 1,4-addition of organotin reagents to cyclic enones. The reactions provided low yields, but high enantioselectivities. Compared with the parent ligand, which achieved up to 84 % ee, the methyl substituent at the allylic position improved the enantioselectivity up to 93 % ee based on steric effects. In summary, the team demonstrated for the first time that chiral trans-cyclooctenes can provide efficient coordination spheres for asymmetric transition-metal catalysis.
- Trans‐Cyclooctenes as Chiral Ligands in Rhodium‐Catalyzed Asymmetric 1,4‐Additions,
Tagui Nagano, Shunsuke Einaru, Kenta Shitamichi, Keisuke Asano, Seijiro Matsubara,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202000956