Frustrated Lewis pairs (FLPs) combine a Lewis-acidic electron-pair acceptor and a Lewis-basic electron-pair donor. The Lewis acid and the Lewis base in an FLP cannot form an adduct due to steric hindrance. FLPs can activate small molecules, most notably H2 and CO2. These reactions are thought to proceed via polar pathways, with heterolytic bond cleavage. However, some evidence of radical formation has been obtained using electron paramagnetic resonance (EPR) spectroscopy. This indicates that single-electron transfer (SET) may play an important role, too.
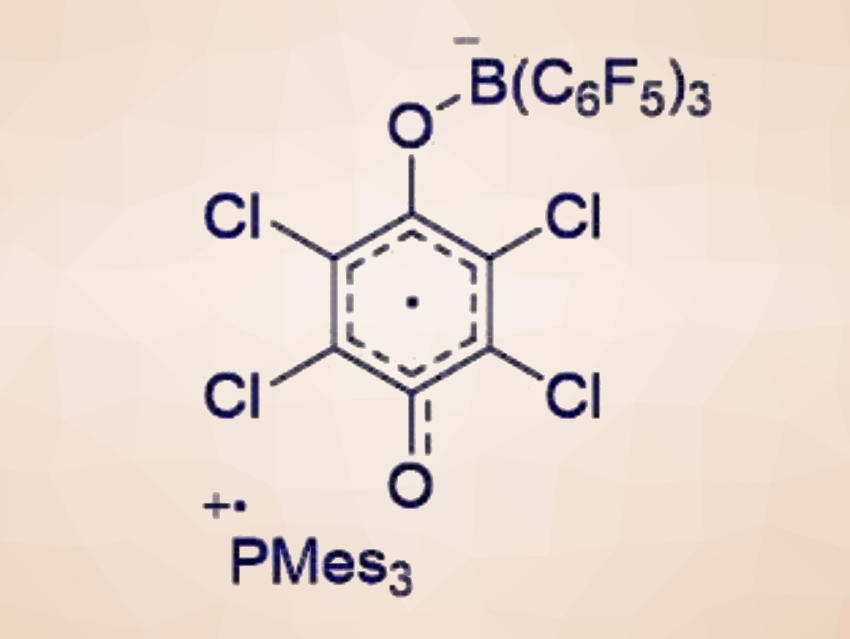
J. Chris Slootweg, University of Amsterdam, The Netherlands, and colleagues have investigated possible radical formations in reactions of FLP systems with dihydrogen (H2), triphenyltin hydride (Ph3SnH), or tetrachloro-1,4-benzoquinone (TCQ), using both experiments and computational methods. The team found that reactions of P/B-based FLP systems with H2 or Ph3SnH do not proceed via a radical pathway. However, EPR spectroscopy of the reaction of a PMes3/B(C6F5)3 FLP system (Mes = 2,4,6-trimethylphenyl) with TCQ showed that these transformations operate via a stepwise radical pathway.
An adduct formation between the Lewis acid B(C6F5)3 and TCQ activates the substrate for SET. Then, a reaction with the Lewis base provides the corresponding radical ion pair, which can undergo coupling to form the product (possible reaction pathways pictured below).
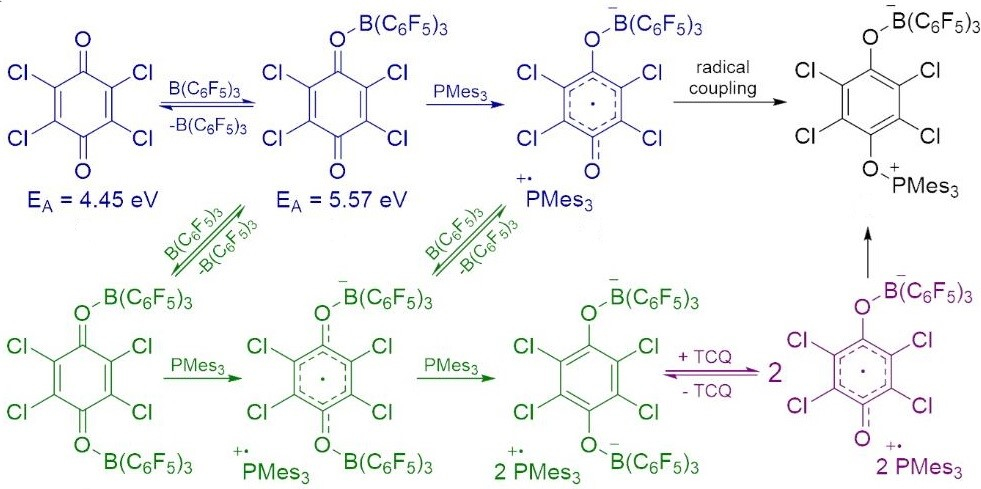
This work demonstrates that within FLP chemistry, both polar and radical pathways can be accessed and shows that the latter generally proceeds via Lewis-acid coordination prior to radical formation.
- Single‐electron Transfer in Frustrated Lewis Pair Chemistry,
Chris Slootweg, Flip Holtrop, Andy Jupp, Bastiaan Kooij, Klaas van Leest, Bas de Bruin,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202009717