Core-modified expanded porphyrins could have uses in non-linear optics, photodynamic therapy, and ion recognition. Expanded porphyrins have more than 18 conjugated π-electrons in their core, which leads to interesting electronic and structural properties.
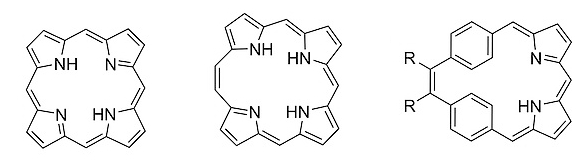
The simplest expanded, conjugated analog of “normal” porphyrin (pictured below on the left) is [20π]‐homoporphyrin (pictured below in the middle). Benziporphyrins are another class of porphyrin analogs, in which one or more pyrrole rings are replaced by a benzene ring. Depending on how the benzene ring is connected within the macrocycle, benziporphyrins are classified as either para‐benziporphyrins or meta‐benziporphyrins. Combining features of homoporphyrins and benziporphyrins leads to a new class of porphyrinoids, called dibenzihomoporphyrins. meso‐Substituted benzihomoporphyrins of the type pictured below on the right had been unknown so far.
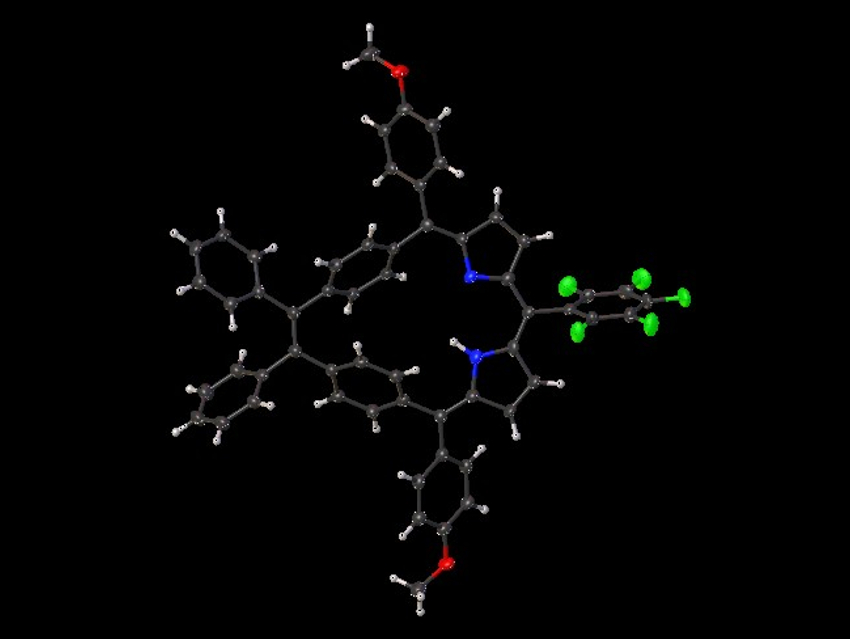
Mathias O. Senge, The University of Dublin and Trinity College Dublin, Ireland, and Technical University of Munich, Garching, Germany, and colleagues have synthesized meso‐substituted di(p‐benzi)homoporphyrins and di(m‐benzi)homoporphyrins that contain phenyl or methyl substituents at the vinylene bridge and aryl substituents at the meso positions. The team used a McMurry coupling reaction and an acid-catalyzed condensation of a dipyrrole intermediate with an aryl aldehyde as key steps in the preparation of the target molecules. The protocol shows good functional-group tolerance and allows access to complex porphyrin architectures.
The synthesized macrocycles are non‐aromatic due to their non‐planarity and the lack of effective π‐conjugation (example structure pictured at the top). Porphyrins and related compounds are often singlet oxygen‐generating photosensitizers. The team tested the products for the possibility to generate singlet oxygen species. Derivatives with pentafluorophenyl or anthracene substituents at the meso positions produced high levels of singlet oxygen, indicating their potential for use in photodynamic therapy.
- Synthesis and Structure of meso‐Substituted [20π]‐Dibenzihomoporphyrins,
Nitika Grover, Ganapathi Emandi, Brendan Twamley, Bhavya Khurana, Vincent Sol, Mathias O. Senge,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202001165