Creating complex polycyclic molecules from simple building blocks often is challenging and requires many reaction steps. Transition-metal-catalyzed C–C coupling reactions, such as the catalytic asymmetric dearomatization (CADA) reaction, are useful tools to synthesize complex molecules efficiently. Homogenous gold catalysis has made significant progress in recent years, but gold has been rarely used in CADA reactions compared with other transition metals.
Gebhard Haberhauer, University of Duisburg-Essen, Essen, Germany, and colleagues have developed a gold(I)-catalyzed one-pot synthesis of complex polycycles via a dearomatization and an allene-diene-alkyne coupling (pictured below). Starting from an o,o-dimethyl-substituted propargyl ether and different alkynes, the team synthesized polycycles using [JohnPhosAu(NCMe)]SbF6 as a gold(I) catalyst. JohnPhos is a bulky biaryl phosphine ligand. The reaction tolerates a wide range of alkyne substrates, and the researchers obtained yields up to 77 %.
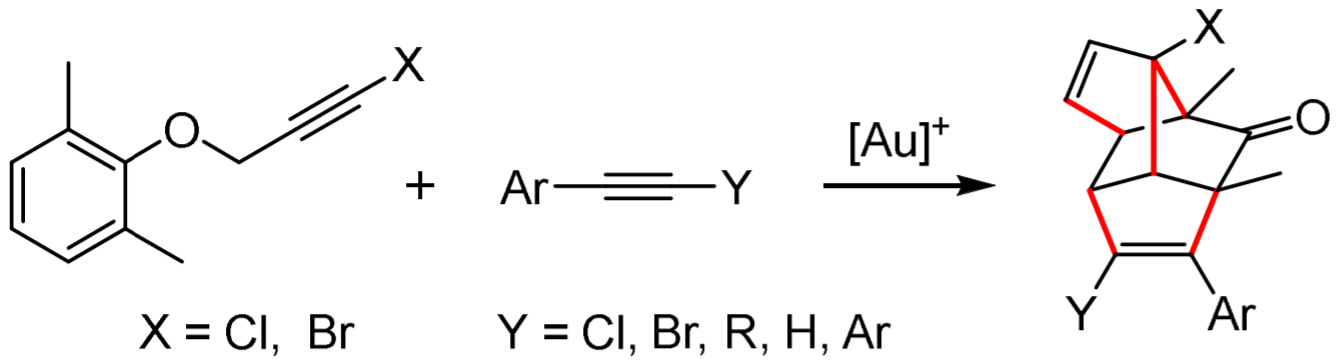
The mechanism can be described as a cascade reaction in two steps: First, an allene is formed by gold(I)-catalyzed dearomatization. Then, the desired product is obtained by an allene-diene-alkyne coupling. Overall, five new C–C bonds are formed in this one-pot reaction (pictured in red). The reaction could be a basis for new syntheses of cyclic and polycyclic molecules.
- Gold(I)‐Catalyzed Allene‐Diene‐Alkyne Coupling Reaction to Polycycles,
Nina Semleit, Mathis Kreuzahler, Gebhard Haberhauer,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202001190


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
