The reductive homologation of CO has been studied since at least 1834, when Justus von Liebig reacted molten potassium and CO gas to give a dark, ill-defined solid. Later, his product—”potassium carbonyl” or (KCO)n—was shown to contain a mixture of species, including potassium benzenehexolate, K6C6O6. The mechanism of this reaction is still unknown, but it has been proposed that transition-metal impurities in the molten potassium promote the formation of K6C6O6.
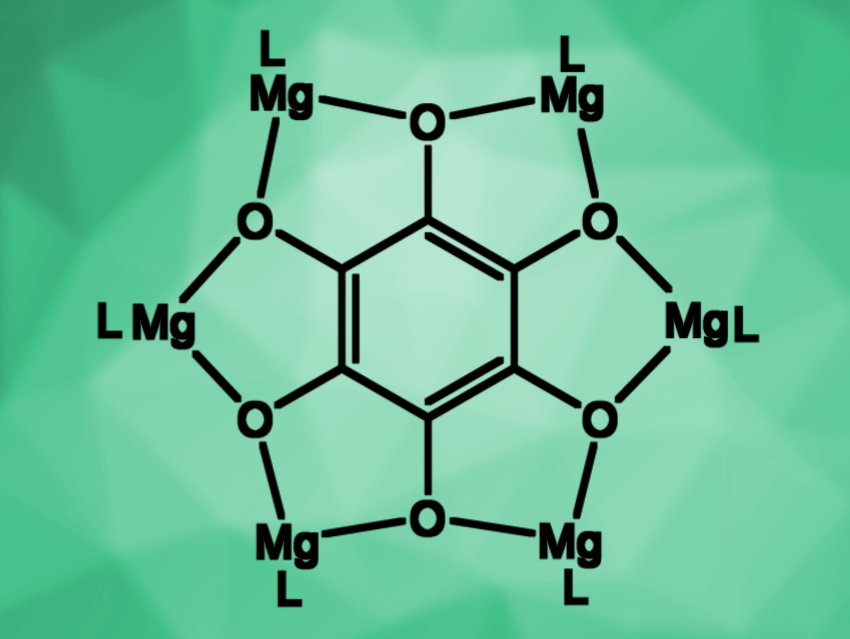
Cameron Jones, Monash University, Melbourne, Australia, Laurent Maron, University of Toulouse, France, and colleagues have shown that soluble, reducing magnesium(I) compounds and Mo(CO)6 can enable the reductive hexamerization of CO and the formation of well-defined magnesium benzenehexolate compounds (reaction pictured below). The team reacted magnesium(I) compounds of the type [{(ArNacnac)Mg}2] (ArNacnac = [HC(MeCNAr)2]–, Ar = mesityl or o-xylyl) with CO in the presence of [Mo(CO)6]. The mesityl and the o-xylyl derivatives were obtained in yields of up to 25 % and 30 %, respectively.
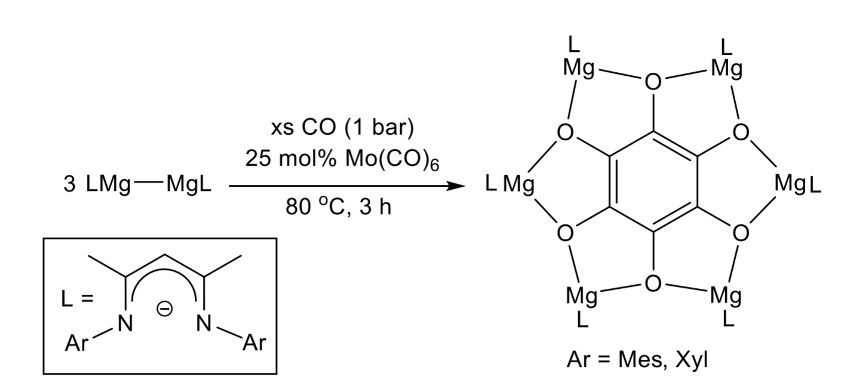
Density functional theory (DFT) calculations and spectroscopic studies showed that the reaction is catalyzed by Mo(CO)6. The work sheds light on the mechanism of Liebig’s classical reaction and could help to understand how other C-C bond forming reactions that use CO as a C1 feedstock operate, such as the Fischer–Tropsch process.
- Reductive Hexamerization of CO Involving Cooperativity Between Magnesium(I) Reductants and [Mo(CO)6]: Synthesis of Well‐Defined Magnesium Benzenehexolate Complexes,
Cameron Jones, Albert Paparo, K. Yuvaraj, Aidan J. R. Matthews, Iskander Douair, Laurent Maron,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202009523