Self-assembly is a powerful tool for the efficient creation of complex molecules. Disulfides, for example, can self-assemble from thiol building blocks via metal- or metalloid-assisted iodine oxidation. Disulfide bonds possess the dynamic ability to break and reform rapidly under suitable conditions, which can, e.g., be used for the synthesis of macrocycles.
Darren W. Johnson, University of Oregon, Eugene, USA, and colleagues have used self-assembly to form disulfide (R–S–S–R) cyclophanes (pictured below on yellow) by treating thiols (R–SH) and a pnictogen salt (e.g., SbCl3) with iodine. Sulfur extrusion using hexamethylphosphorous triamide (HMPT) removes one S atom from each disulfide group to form more stable thioether (R–S–R) cyclophanes (pictured below on blue). Removal of the remaining S atoms via a photochemically promoted route using triethyl phosphite gave hydrocarbon cyclophanes (pictured below on green), which are analogues of the well-known [n]-paracyclophanes.
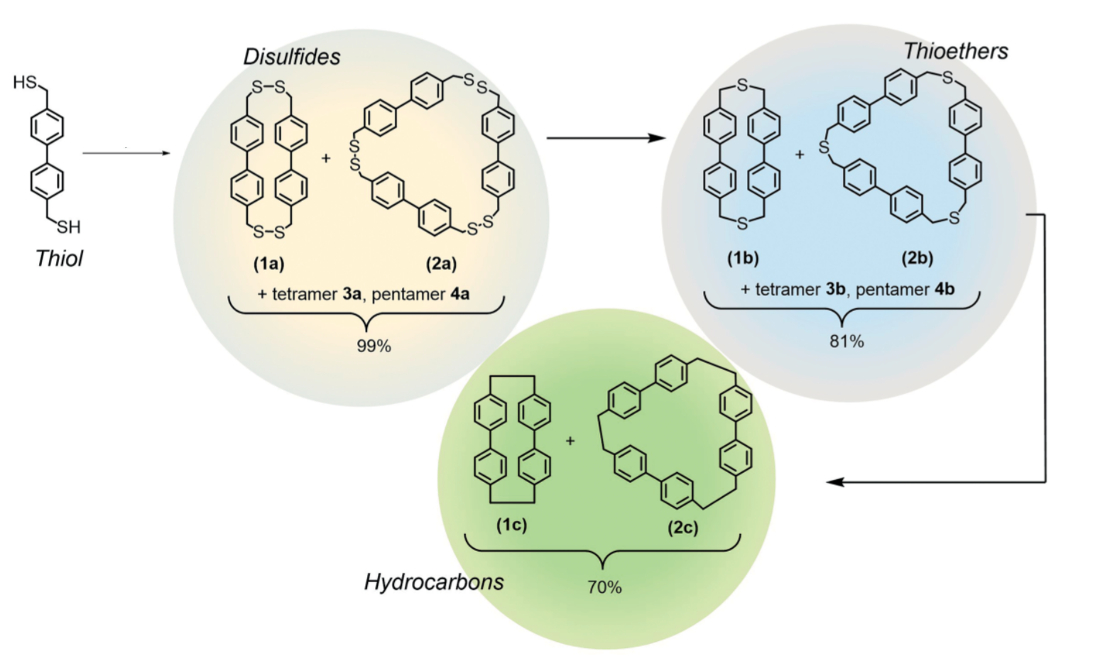
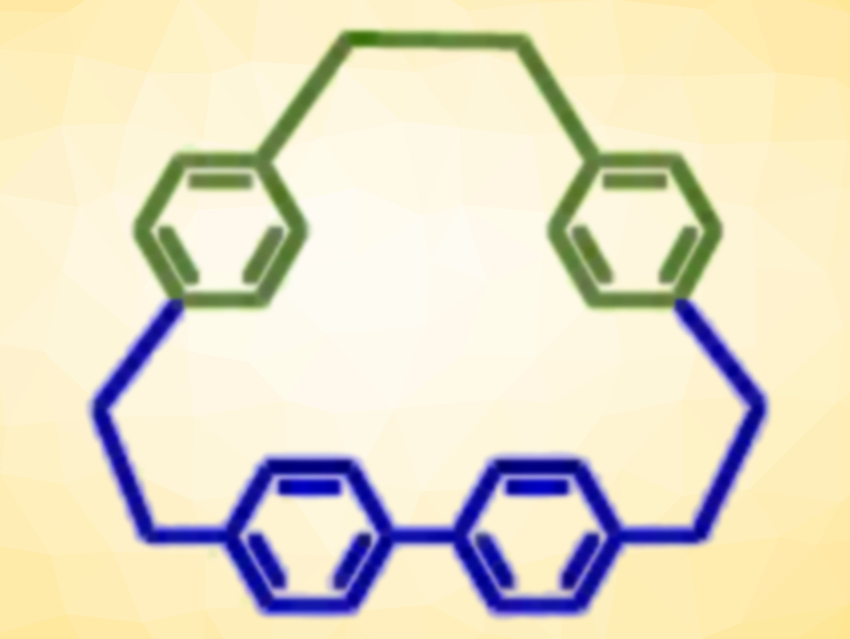
The team found that they could also mix different thiols to make lower symmetry disulfides (R–S–S–R’). Sulfur removal gave new unsymmetrical thioethers (R–S–R’). The team then used sulfur photo-extrusion to form unsymmetrical hydrocarbon macrocycles (example pictured at the top).
- An Efficient Route to Symmetrical and Unsymmetrical Disulfide, Thioether, and Hydrocarbon Cyclophanes,
Ngoc‐Minh Phan, Trevor A. Shear, Lev N. Zakharov, Darren W. Johnson,
Eur. J. Org. Chem. 2020.
https://doi.org/10.1002/ejoc.202001242



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)