Saccharides are biologically important compounds. Saccharide recognition is a challenging topic in the field of host-guest chemistry due to their structural complexity. Helical foldamers, i.e., chain-like molecules that fold into ordered helices, can be suitable for saccharide recognition because they can effectively form hydrogen-bonding networks with multiple hydroxy groups of saccharides. The affinity and selectivity of such foldamers can be tuned by changing their structure.
Yuki Ohishi, Toshikazu Takata, and Masahiko Inouye, University of Toyama (Japan) have developed a pyridine–acetylene–aniline oligomer that forms a helical foldamer for saccharide recognition. This array can effectively form hydrogen bonds with saccharide hydroxy groups via its pyridine and aniline rings. The oligomer was synthesized using consecutive Sonogashira reactions.
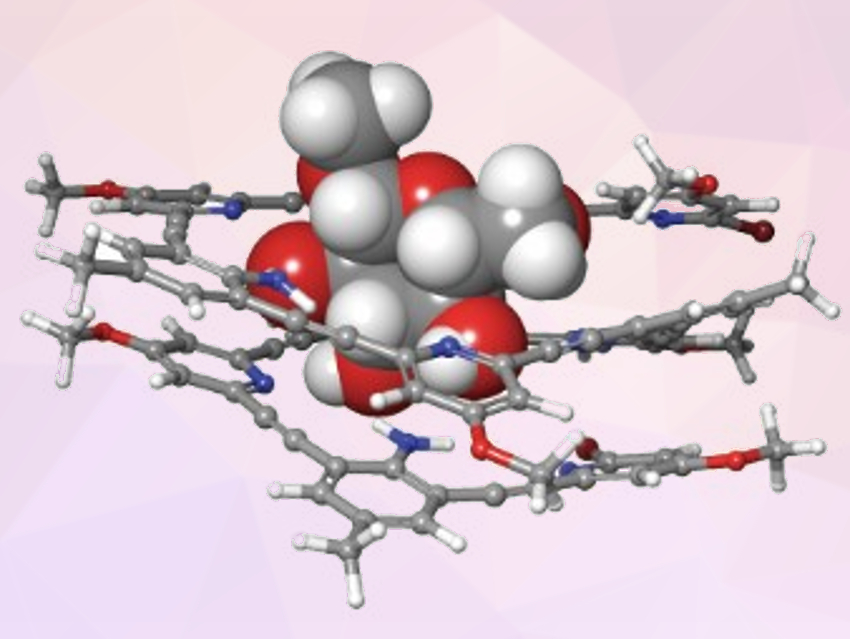
The oligomer associates with saccharides to form helical complexes (example pictured) in apolar solvents. It is relatively stable under basic conditions, which could make it useful for derivatization reactions of saccharides. To demonstrate this, the team prepared a 4-(dialkylamino)pyridine (DMAP)-functionalized pyridine–acetylene–aniline oligomer, which catalyzed the acylation of a glycoside. According to the researchers, regioselective catalysts could be created by changing the connection sites between the oligomer recognition array and the DMAP groups.
- A Pyridine−Acetylene−Aniline Oligomer: Saccharide Recognition and Influence of this Recognition Array on the Activity as Acylation Catalyst,
Yuki Ohishi, Toshikazu Takata, Masahiko Inouye,
ChemPlusChem 2020.
https://doi.org/10.1002/cplu.202000603


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
