Salts of hexafluoridosilicic acid have been known since the beginning of the 19th century, with K2SiF6 being first mentioned in 1882. However, a detailed structural description is limited to alkali metal hexafluoridosilicates and some other hexafluoridosilicates. Hubert Huppertz, University of Innsbruck, Austria, and colleagues have elucidated the structures of KLiSiF6 and CsLiSiF6 using single crystal diffraction.
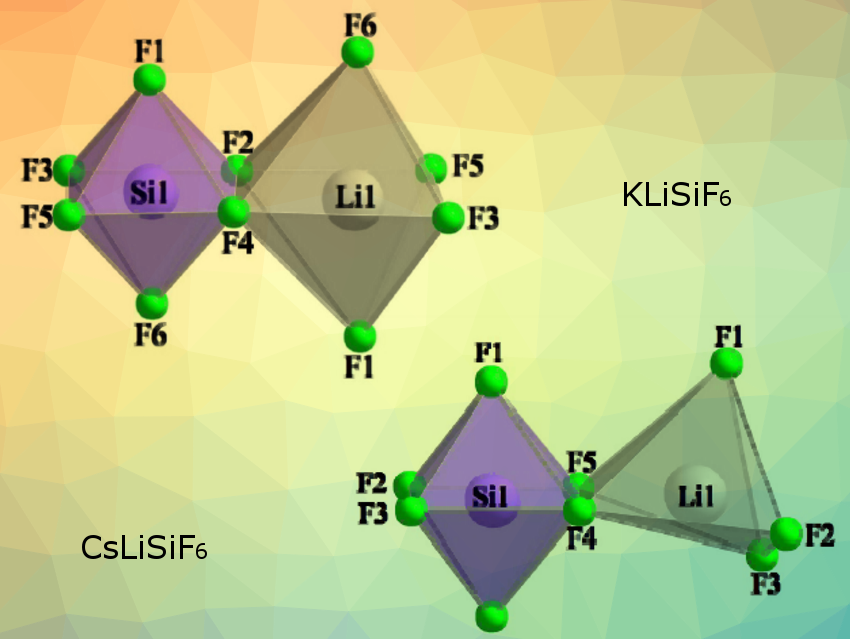
The researchers synthesized KLiSiF6 and CsLiSiF6 via a high‐pressure/high‐temperature synthesis route. They found that both substances crystallize in the orthorhombic crystal system with space group Pbcn (no. 60), but show different crystal structures: KLiSiF6 is isostructural to (NH4)MnFeF6, whereas CsLiSiF6 forms its own new structure type.
The main motifs of KLiSiF6 are an [SiF6]2− and an [LiF6]5− unit. In contrast, the main motifs of CsLiSiF6 are an [SiF6]2− and an [LiF5]4− unit (pictured). Within both substances these units are interconnected and form 3‐dimensional networks.
The units are interesting for partial substitution with [MnF6]2− units. Mn4+‐doped KLiSiF6 is a red luminescent material. KLiSiF6:Mn4+ shows a red luminescence with a maximum emission wavelength of 631 nm.
- KLiSiF6 and CsLiSiF6 − A Structure Investigation,
Christiane Stoll, Markus Seibald, Dominik Baumann, Jascha Bandemehr, Florian Kraus, Hubert Huppertz,
Eur. J. Inorg. Chem. 2020.
https://doi.org/10.1002/ejic.202000867