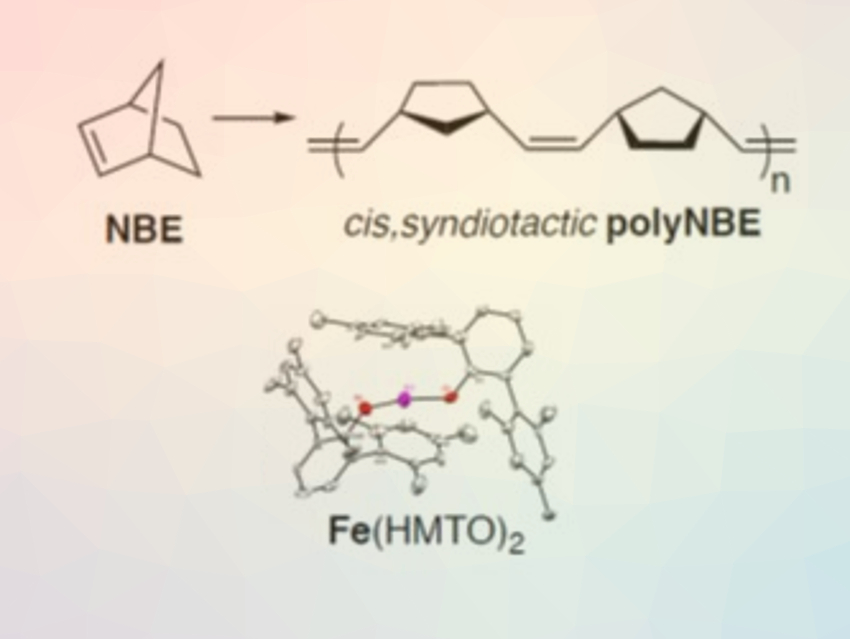
Olefin metathesis is widely used to produce plastics, household chemicals, agrochemicals, and pharmaceutical drugs. Usually, catalysts for olefin metathesis are based on rare metals, such as ruthenium, molybdenum, and tungsten. Catalysts based on abundant metals with low toxicity could be less expensive and greener alternatives. Theoretical studies have indicated that iron complexes could promote olefin metathesis, but no successful examples of iron‐catalyzed olefin metathesis had been reported so far.
Konstantin V. Bukhryakov, Florida International University, Miami, USA, and colleagues have found that a low-coordinate iron bisalkoxide is the first iron-based catalytic system capable of performing the ring-opening metathesis polymerization (ROMP) of norbornene (NBE) with moderate activity and high selectivity. The catalyst is a two‐coordinate homoleptic iron complex, Fe(HMTO)2 [HMTO = O‐2,6‐(2,4,6‐Me3C6H2)2C6H3] (pictured above). By using inductively coupled plasma mass spectrometry (ICP-MS), the researchers eliminated the possibility that trace quantities of foreign metals might cause the observed catalytic activity.
The team found that the use of heteroleptic Fe(HMTO)(RO) (RO = (CH3)2CF3CO, CH3(CF3)2CO, or Ph(CF3)2CO), prepared in situ, significantly increases the rate of the reaction compared with Fe(HMTO)2 while preserving stereoselectivity. According to the researchers, the nature of the active catalyst remains unclear and requires further investigation. The reaction may involve polynuclear iron clusters and/or a yet unknown mechanism.
- Stereospecific Ring‐Opening Metathesis Polymerization of Norbornene Catalyzed by Iron Complexes,
Dmitry S. Belov, Logesh Mathivathanan, Melanie J. Beazley, William Blake Martin, Konstantin Bukhryakov,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202011150