Water oxidation is an important reaction for the production of clean, sustainable fuels. In nature, the reaction is promoted by the oxygen-evolving complex in photosystem II, in which a catalytic center is surrounded by hole-transporting amino acid residues. However, in artificial systems, there had been no example of a water oxidation system that has a catalytic center surrounded by hole transporters until now.
Mio Kondo, Shigeyuki Masaoka, Osaka University, Japan, and colleagues have developed a catalyst system for water oxidation that integrates these two functions. The team first prepared a precursor that contains a tetranuclear, cubane-like cobalt moiety as the catalytic center, surrounded by carbazole ligands (pictured below). Via an electrochemical polymerization, the carbazole units were then dimerized to biscarbazole units, which can act as hole transporters.
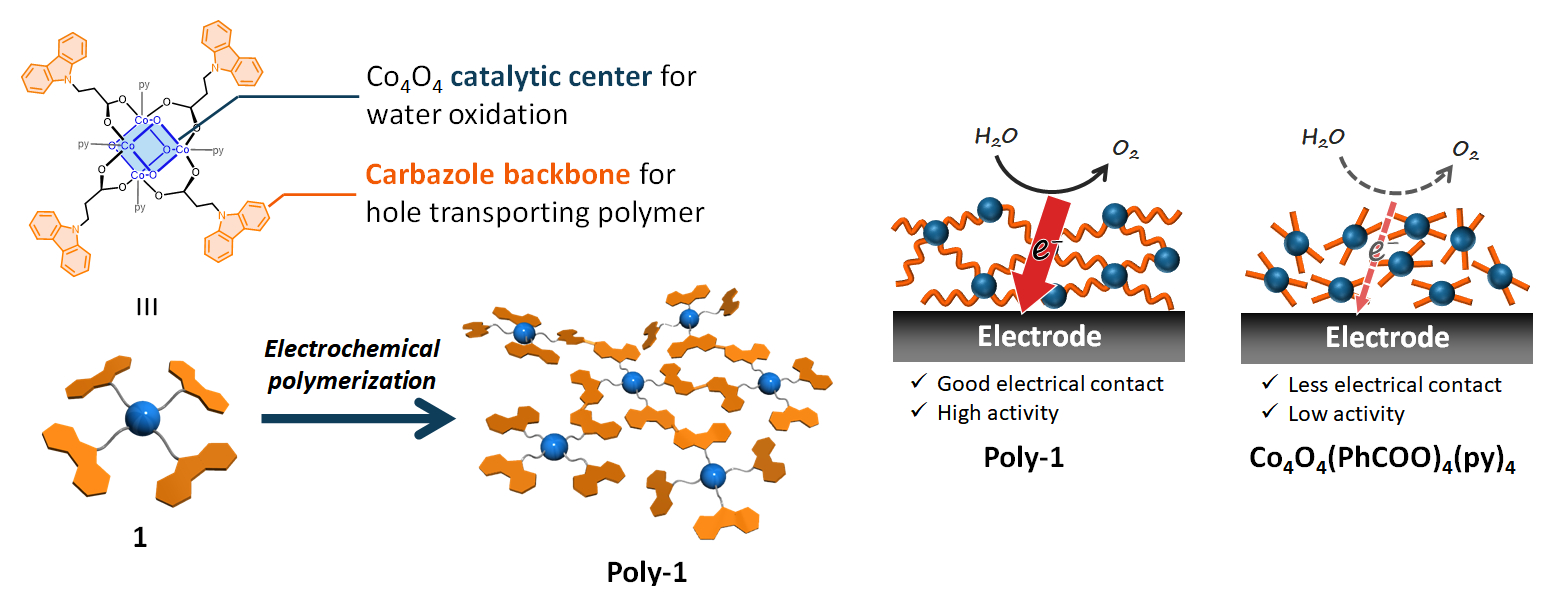
The resulting polymer can catalyze the water oxidation reaction with a high faradaic efficiency and a low overpotential. The hole-transporting ability of the biscarbazole units proved essential for efficient water oxidation. A cobalt complex without biscarbazole moieties showed only low activity. These results demonstrate the importance of the integration of catalytic centers and hole-transporting units and offer a new strategy for constructing artificial water oxidation systems.
- Electrochemical Polymerization Provides a Function-Integrated System for Water Oxidation,
Hikaru Iwami, Masaya Okamura, Mio Kondo, Shigeyuki Masaoka,
Angew. Chem. Int. Ed. 2020.
https://doi.org/10.1002/anie.202015174