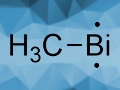Priv.-Doz. Crispin Lichtenberg, University of Würzburg, Germany, completed his habilitation last year. His research has already received several highly respected awards. Here he talks with ChemistryViews about his fascination with bismuth chemistry and rewarding moments in research.
What got you interested in bismuth chemistry?
One point was that bismuth chemistry, at least from my perspective, was not very well researched. This is not to say that there have not been very many researchers who did their best and revealed fascinating results. But compared to other fields of research within chemistry, I felt that bismuth chemistry was sort of underdeveloped. More importantly, I saw a high potential for future developments in innovative and selective bond activation processes.
Do you have an idea why bismuth chemistry used to be less in the focus of research efforts?
I can only speculate, of course. I assume that part of the reason was that main-group chemistry, in general, was not really fashionable or trendy for some time. As main-group chemistry was revived within the last decades, one thriving goal was to generate main-group–main-group multiple bonds, so something like a tin–tin double bond. It seemed to be a little bit easier and more obvious to go for group 14 elements in the beginning. I assume that may have contributed to what I call a slight underdevelopment of bismuth chemistry.
What makes bismuth so interesting for you?
When you look at bismuth as an element, you find it pretty much at the bottom right of the periodic table, which means it really is a heavy element. It is the heaviest non-radioactive element – or more correct: You can ignore its radioactivity because it is so minimal.
Thus, bismuth brings along all the properties that you can expect from a heavy element. That is, it shows relativistic effects, it has very large and polarizable atomic orbitals. As a result, bismuth complexes can be described as having relatively low bond association energies, so you can break bismuth–element bonds – for example, a bismuth–carbon bond – relatively easy. That, of course, makes it possible to start chemical reactions under relatively moderate conditions. That is one thing we try to exploit.
Also, when you have a bond-dissociation event – in other words, when you break (to stay with the example) a Bi–C bond — you have to invest relatively little energy. That means that this bond dissociation can become reversible. So re-association is also possible. This is something that makes these kinds of bond-dissociation/bond-association events very interesting for catalytic applications.
Can you say a bit more about your research? I saw your publication on BiCH3.
We have focused our research on molecular chemistry. We aim to create well-defined molecular bismuth complexes. This is what our expertise is, if you will. We focus on that to exploit the sort of element-specific properties of bismuth.
BiCH3 is a very simple molecule with two functional groups, namely, one methyl group and one bismuth atom. We were able to detect this molecule in cooperation with other researchers. From my perspective, this represented a great example of cooperative research efforts from the University of Würzburg.
Association energies of bismuth compounds are usually relatively low. We wanted to investigate this for a very simple molecule, namely, trimethylbismuth. So we started from trimethylbismuth and heated that compound under certain conditions. In doing so, we were able to find conditions where we can abstract one methyl group with relatively high selectivity. That gives a dimetalbismuth radical.
We can also abstract a second metal group. That gives the compound that you mentioned, which is also the title compound of that work: methylbismuth [1]. Methylbismuth has two unpaired electrons. This was the first time that an organometallic biradical reactive intermediate of bismuth could be created, detected, and characterized.
In a second step, we were interested not only in creating and investigating this molecule but also in seeing how it reacts. For that, we designed a, let’s say, control reaction. We found bismuth-carbon bond hemolysis takes place under moderate conditions in solution, and we found at least clues that this methylbismuth can be created and selectively inserted into a substrate in the condensed phase.
This was, I would say, a very fundamental piece of research. In this work, we did not yet describe any catalytic application. But I see this as a starting point, or, I should probably say a bit more cautiously, I hope this will be a starting point for the development of a richer bismuthinidene chemistry and for exploiting highly reactive bismuthinidenes in chemical synthesis and catalysis.
You have started a research project called “Bismuth goes radical”. Can you say something about that or is it still at an early stage?
I cannot give too many details because it really just started, but I can give the general idea. That is probably captured within the title itself: In this project, we want to focus on the radical chemistry of bismuth.
What I said earlier about the bond-dissociation energy is also one crucial point of this project. We want to address the selective homolytic bond dissociation of bismuth X bonds. This means we have a bismuth atom bound to some other atom; to illustrate, we can stick to the example of the carbon atom again. Then we want to cleave that bond homolytically. One electron then goes to the carbon atom and one electron to the bismuth atom, and that gives us a pair of radicals. We want to exploit the chemistry of the radicals that we can generate this way.
Again, all the things that I have mentioned earlier also come into play here. We hope to generate radicals under relatively mild conditions, and we hope to generate them in, say, a reversible manner or in a manner where reversibility can play a role. This in turn opens up opportunities to enter catalytic scenarios.
What do you enjoy most about your research?
First of all, it is always a team effort. I am not doing the research myself; I am contributing to it. That means I always work together with undergraduate students, Ph.D. students, postdocs, and collaboration partners. Working with these people can be very enjoyable.
Within that context, I think two things can be really rewarding. One is when a plan really works. That maybe sounds a bit strange, but everybody who has studied chemistry probably knows that things do not work as expected a lot of times when you are conducting research. When a plan that a team has dynamically developed for a long time actually works, it can be really rewarding.
But the second thing that can be really great is the opposite of that. When you find out that something does not work the way you expected but instead you discover something really unexpected and can make use of it, I think that is also especially rewarding.
I think this is basically the fascinating twist to science. You always have to keep your eyes open and keep your mind open and analyze what you find.
Being open to what is in front of you, not seeing what you want to see. A very important quality for successful research. Do you think that this can be learned?
I think some people are more skilled than others in keeping an eye open to unexpected results. But this is definitely something that can be learned. As with most things in life, I guess some people tend to enjoy this and that is why they are good at it.
What inspired you most in chemistry or during your career?
I don’t think I can name just one event. What always gave me a certain drive was again something that I have mentioned before, and that is the rewarding moments in science. So when you come across something that you think is really unusual and a great scientific finding. That does not mean that it has to be a great scientific achievement overall; it’s just that it changes your view of the molecules you are working with. And it changes the small world that you are dealing with as a researcher. These small-scale, world-changing moments are very eye-opening and rewarding for a scientist.
What would you like to be doing in ten years?
I would hope to still be working with inspiring students and still making exciting discoveries in chemistry. Of course, it is very hard to predict how exactly chemical sciences will develop, but I hope to be part of that development. It would be great to use bismuth chemistry in helping to develop new, broadly applied reactions in academic and industrial synthetic chemistry.
Besides your research, what are you doing in your spare time?
The sad part is that my spare time has definitely become less and less. The part of my life that is not directly connected to work I spend with my friends and family. That is very important to me. As a hobby, I do enjoy sports, traveling, and reading whenever I find time to do so.
Thank you for the interview.
Reference
[1] Deb Pratim Mukhopadhyay, Domenik Schleier, Sara Wirsing, Jacqueline Ramler, Dustin Kaiser, Engelbert Reusch, Patrick Hemberger, Tobias Preitschopf, Ivo Krummenacher, Bernd Engels, Ingo Fischer, Crispin Lichtenberg, Methylbismuth: an organometallic bismuthinidene biradical, Chem. Sci. 2020, 11, 7562–7568. https://doi.org/10.1039/D0SC02410D
Crispin Lichtenberg, born 1984, studied chemistry in Marburg, Germany, and Cambridge, UK. He received his Ph.D. from RWTH Aachen, Germany, in 2013 and worked as a postdoc at ETH Zurich, Switzerland, with Hansjörg Grützmacher until 2015. Since 2016, he has pursued independent research under Holger Braunschweig at the University of Würzburg, Germany, with the support of a Liebig Fellowship from the Chemical Industry Fund. He completed his habilitation in 2020 and since the beginning of 2020 he has been working as a Privatdozent at the Faculty of Chemistry and Pharmacy.
His research focuses on cationic and low-valent bismuth species used as reagents and catalysts in synthetic chemistry. These species allow the specific targeting of polar or radical reaction pathways, including the use of new redox-active ligands. Unusual reactivities and selectivities can thus be achieved, e.g., in the areas of CH activation, radical coupling, and activation of small (reaction-carrying) molecules.
Selected Awards
- December 2020: Ernst Haage Prize for Chemistry
- September 2020: Starting Grant of the European Research Council
- May 2020: Lecturer Award of the Fonds der Chemischen Industrie (FCI)
Selected Articles
- B. Ritschel, J. Poater, H. Dengel, F. M. Bickelhaupt, C. Lichtenberg, Double CH Activation of a Masked Cationic Bismuth Amide, Angew. Chem. Int. Ed. 2018, 57, 3825–3829. https://doi.org/10.1002/anie.201712725
- J. Ramler, J. Poater, F. Hirsch, B. Ritschel, I. Fischer, F. M. Bickelhaupt, C. Lichtenberg, Carbon Monoxide Insertion at a Heavy p-Block Element: Unprecedented Formation of a Cationic Bismuth Carbamoyl, Chem. Sci. 2019, 10, 4169–4176. https://doi.org/10.1039/C9SC00278B
- J. Ramler, I. Krummenacher, C. Lichtenberg, Bismuth Compounds in Radical Catalysis: Transition Metal Bismuthanes Facilitate Thermally-Induced Cyclo-Isomerizations, Angew. Chem. Int. Ed. 2019, 58, 12924–12929. https://doi.org/10.1002/anie.201904365
- D. P. Mukhopadhyay, D. Schleier, S. Wirsing, J. Ramler, D. Kaiser, E. Reusch, P. Hemberger, T. Preitschopf, I. Krummenacher, B. Engels, I. Fischer, C. Lichtenberg, Methylbismuth: an Organometallic Bismuthinidene Biradical, Chem. Sci. 2020, 11, 7562–7568. https://doi.org/10.1039/D0SC02410D
- K. Oberdorf, A. Hanft, J. Ramler, I. Krummenacher, F. M. Bickelhaupt, J. Poater, C. Lichtenberg, Bismuth Amides Mediate Facile and Highly Selective Pn-Pn Radical Coupling Reactions (Pn = N, P, As), Angew. Chem. Int. Ed. 2021. https://doi.org/10.1002/anie.202015514
- A. Hanft, K. Radacki, C. Lichtenberg, Cationic Bismuth Aminotroponiminates: Charge Controls Redox Properties, Chem. Eur. J. 2021. https://doi.org/10.1002/chem.202005186
Also of Interest
News: Free Methylbismuth Synthesized
ChemistryViews 08 June 2020
First non-stabilized organometallic bismuthinidene generated in the gas phase