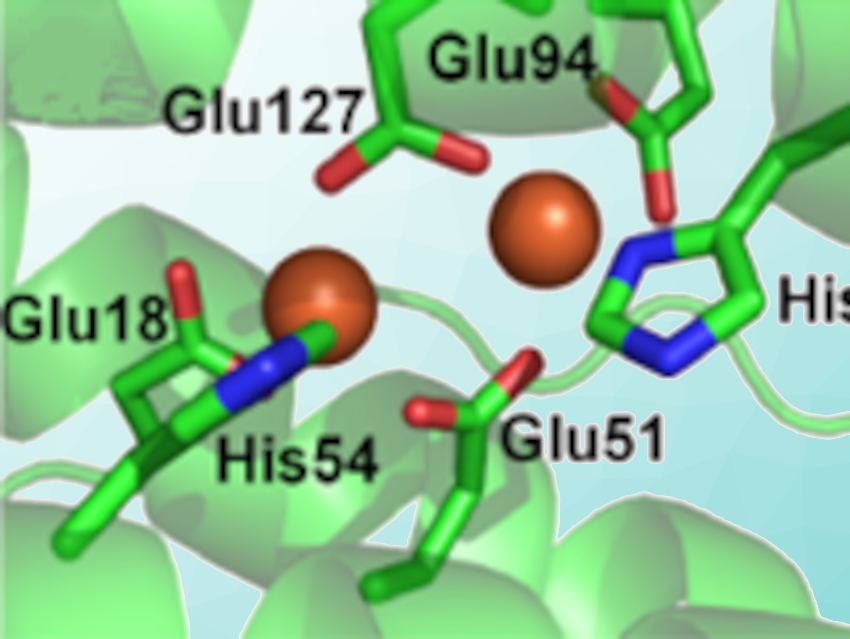
Ferritins are proteins that remove potentially toxic iron from solution and deposit it within their internal cavity for future use. Bacterioferritin from the gut bacterium Escherichia coli (pictured) belongs to a subclass of ferritins that contain haem. The role of the haem had not been clear for a long time, but it was recently shown to be related to the release of iron back into solution when cellular iron levels drop. To deposit iron inside the protein’s shell, ferritins oxidize water-soluble iron ions. They can use both oxygen and hydrogen peroxide to do so.
Dimitri A. Svistunenko, Michael T. Wilson, University of Essex, UK, and colleagues have found that H2O2 is about 1,000 times more efficient in this process than O2, which makes the bacterioferritin extremely efficient in removing hydrogen peroxide. To study this phenomenon, the researchers used UV-Vis, electron paramagnetic resonance (EPR), and Mössbauer spectroscopies to follow the reactions when bacterioferritin, pre-loaded with Fe2+, was exposed to O2 or H2O2. Using UV/Vis spectroscopy and kinetic experiments, the team also discovered how the haem group might play a key role in this detoxification process—i.e., acting as an electron source for regeneration of the protein’s active site.
These findings change the way the researchers view the protein’s primary function: It is not really about collecting iron, but about using iron to reduce harmful hydrogen peroxide to water. The discovery of this role for haem in the protein opens up a whole new avenue for the exploration of bacterioferritins.
- Iron oxidation in Escherichia coli bacterioferritin ferroxidase centre, a site designed to react rapidly with H2O2 but slowly with O2,
Jacob Pullin, Michael T. Wilson, Martin Clémancey, Geneviève Blondin, Justin M. Bradley, Geoffrey R. Moore, Nick E. Le Brun, Marina Lučić, Jonathan A. R. Worrall, Dimitri A. Svistunenko,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202015964 - Electron transfer from haem to the di‐iron ferroxidase centre in bacterioferritin,
Jacob Pullin, Justin M. Bradley, Geoffrey R. Moore, Nick E. Le Brun, Michael T. Wilson, Dimitri A. Svistunenko,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202015965