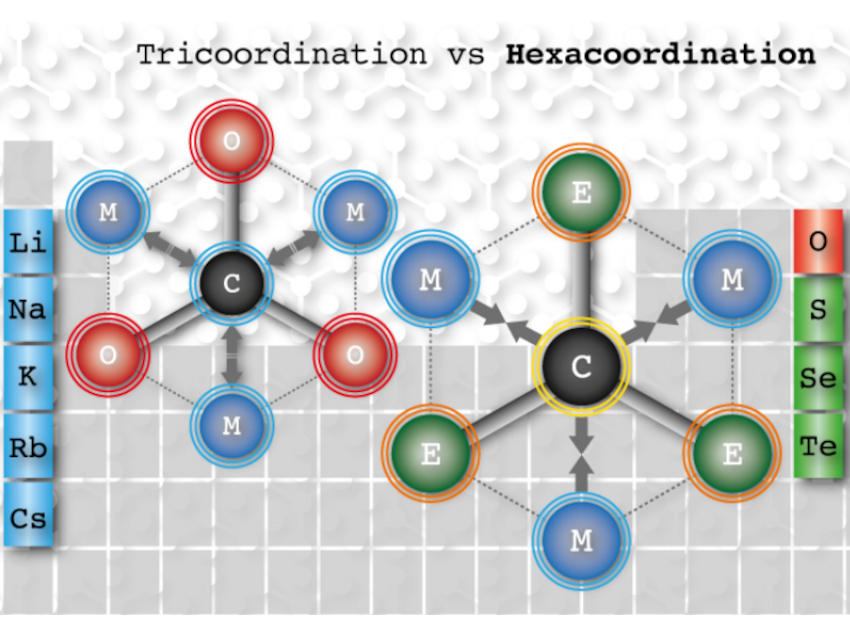
Any introductory organic chemistry course includes the statement that a tetracoordinate carbon has a tetrahedral shape. However, in 1970, Roald Hoffmann et al. suggested rules to stabilize a planar tetracoordinate carbon (ptC) [1]. These findings inspired the design and synthesis of ptCs, and were later extended to planar pentacoordinate carbon (ppC). However, the higher the coordination number, the more elusive these species become. So far, only a handful of ppC structures are known, and not a single planar hexacoordinate carbon (phC)—despite researchers’ efforts.
Using state-of-the-art computational chemistry methods, Jorge Barroso, Gabriel Merino, Centro de Investigación y de Estudios Avanzados, Unidad Mérida, México, Alejandro Vásquez‐Espinal, William Tiznado, Universidad Andres Bello, Santiago, Chile, and colleagues have predicted the first species that can be considered a true phC (pictured). The fifteen proposed structures are of the type CE3M3+ (E = S–Te and M = Li–Cs) series. The compounds were designed via a strategy that involved replacing oxygen in the D3h-symmetric CO3Li3+ structure with heavier and less electronegative chalcogens, inducing a negative charge on the C atom and an attractive electrostatic interaction between C and the alkali‐metal cations.
The team found that all global minima of the studied CE3M3+ clusters have a planar D3h symmetry, with C−E distances between a single and a double bond. The carbon achieves hexacoordination by bonding covalently to three chalcogen ligands and electrostatically to the alkali metal ligands, which was confirmed using bonding analysis.
- Planar Hexacoordinate Carbons: Half Covalent, Half Ionic,
Luis Leyva-Parra, Luz Diego, Osvaldo Yañez, Diego Inostroza, Jorge Barroso, Alejandro Vasquez-Espinal, Gabriel Merino, William Tiznado,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202100940
Reference
- [1] Planar tetracoordinate carbon,
Roald Hoffmann, Roger W. Alder, Charles F. Wilcox,
J. Am. Chem. Soc. 2002, 92, 4992–4993.
https://doi.org/10.1021/ja00719a044
Also of Interest
- Event: ChemPhysChem/ChemSystemsChem Joint Virtual Symposium: Computational Chemistry
Presentations by Markus Reiher, Leticia González, and Gabriel Merino