Batteries charge and recharge—apparently all thanks to a perfect interplay of electrode material and electrolyte. However, for ideal battery function, the solid electrolyte interphase (SEI) plays a crucial role. Qiang Zhang, Tsinghua University, Beijing, China, and colleagues have studied nucleation and growth of this layer in atomic detail and found that the properties of anions and solvent molecules need to be well balanced.
The Solid Electrolyte Interphase
In lithium-ion batteries, the SEI forms at the beginning of the first charging process, when a potential is applied. Elements from the electrolyte deposit on the graphite electrode and form a coating that soon covers the entire electrode. Only after this layer is completed can the positive lithium ions intercalate in the electrode without exfoliating the electrode material.
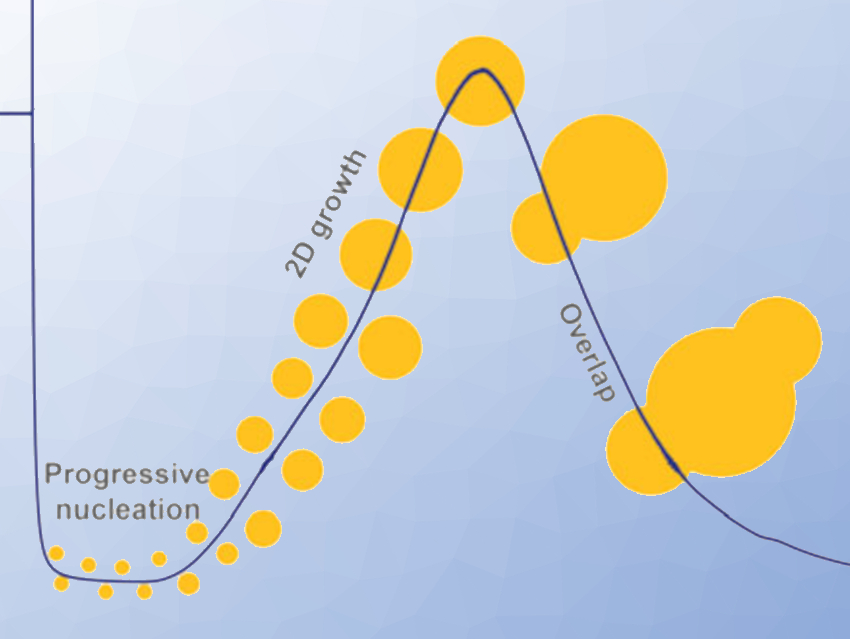
The researchers have taken a closer look at the nucleation and growth of the SEI. The electrolyte in lithium-ion batteries contains lithium salt and a solvent. Strongly solvating solvents wrap the lithium ion, and the anions float freely. In contrast, weakly solvating electrolytes enable a closer attachment of the anions to the lithium ion. Here, the anions remain part of the inner solvation shell.
Anions and Solvents Direct Nucleation and Growth
This inner solvation shell must be stripped off from the lithium to allow SEI formation and growth. The researchers demonstrated that the anions of the inner shell first adsorbed at the fresh electrode and then took up two electrons in an electrochemical reaction. This latter event triggered decomposition and nucleation of the SEI. The team concluded that SEI formation mainly depended on how easily the anions can grab electrons and decompose compared with the solvent.
The scientists used electrochemical techniques and atomic force microscopy (AFM) to investigate the crystal growth until completion of the layer. They found that a smooth layer only formed at low overpotentials. The solvent also influenced the overpotential. The team also noted that solvents having a high affinity to the crystalline layer produced no overpotential at all.
They concluded that future designs of high-performance electrodes should focus more on the interplay between the negative ions of the lithium salt and the solvent. To allow a homogeneous inorganic, crystalline SEI to be formed, the anions should outcompete the solvent; they should more easily adsorb to the electrode surface and undertake electrochemical reactions. In addition, the decomposition products should be solid and insoluble, but still show a certain affinity to the solvent.
- Nucleation and Growth Mechanism of Anion‐Derived Solid Electrolyte Interphase in Rechargeable Batteries,
Chong Yan, Li‐Li Jiang, Yu‐Xing Yao, Yang Lu, Jia‐Qi Huang, Qiang Zhang,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202100494