4H-Cyclopenta[def]phenanthrene (CPP, 1, pictured) is a valuable building block in the production of photoactive materials. These materials have applications, e.g., in blue-colored organic light-emitting diodes (OLEDs) or solar cells. Existing methods for the synthesis of CPP have issues such as lengthy syntheses, poor yields, or a need for hazardous reagents, which makes their scale-up challenging and CPP expensive.
Dmitri V. Filippov, Grégory F. Schneider, Leiden University, The Netherlands, and colleagues have developed a concise, three-step method to synthesize CPP on a gram scale (pictured below). The team started from pyrene 2, which was oxidized to the 4,5‐dione 3. The key step of the synthesis is the formation of oxoCPP 4 via a ring contraction of the dione. This intermediate was sublimated from the reaction mixture in which it was formed and deposited on the inside of the reaction flask as bright yellow crystals. Using a custom reaction vessel, oxoCPP could be obtained in pure form without needing further purification. A final reduction then gave the desired CPP.
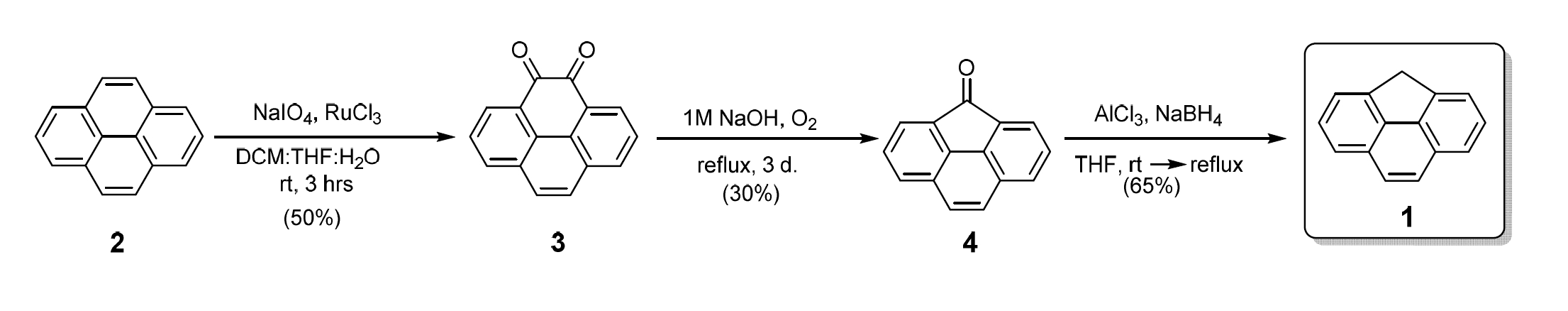
The developed method could make the production of photoactive materials significantly cheaper compared to previously known syntheses. All synthetic steps use relatively cheap and non-hazardous materials, and the two steps which do require additional purification were optimized to minimize the use of solvent. The method is, thus, quick and economical. According to the researchers, it could ultimately also allow the safer scale-up of the production of photoactive materials.
- A Three-Step Synthesis of 4H–Cyclopenta[def]phenanthrene from Pyrene,
Alex van der Ham, Hermen S. Overkleeft, Dmitri V. Filippov, Gregory Schneider,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100190
![Three-step Synthesis of 4H-Cyclopenta[def]phenanthrene from Pyrene](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/178882e749f.jpg)



