Bacterial biofilms can be problematic when they form on sensitive surfaces. When they develop on infected skin, they can lead to chronic wounds and contribute to antibiotic resistance. Jingwei Xie, University of Nebraska Medical Center, Omaha, USA, and University of Nebraska‐Lincoln, USA, and colleagues have developed a biphasic wound dressing that may help combat biofilms in a gentle, but effective way. One side of the patch pierces through the biofilm and releases drugs, while the other side holds a reservoir containing an effective antibiotic cocktail
Biofilms – More than Just a Nuisance
Bacteria are much harder to tackle when they are in the form of biofilms. These assemblies enable them to protect themselves against mechanical attacks, detergents, or drugs by forming polymeric extracellular matrices and other physical and chemical barriers. Biofilms may cover sensitive surfaces such as the inner linings of food tanks, teeth (plaque, what a nuisance!), surgical tools, and wounds, where they are hard to remove.
This is especially problematic in clinical settings. Many pathogenic bacteria have developed multiresistance to antibiotic agents. Protected by their physical and chemical armor, bacteria in biofilms have more time to develop defense responses to any new chemical weapon launched against them. When these biofilms settle in wounds, this can stop the wounds from healing. As a last resort, physicians often have to remove the affected areas mechanically, which is painful for the patient and does not guarantee that the film will not resurface again.
To help those patients, the researchers developed a multilevel approach that works similarly to how nicotine patches release their drug through the skin and into the body. The researchers designed a mechanical scaffold that ensures permanent physical contact with the biofilm and, at the same time, disrupts its protective matrices. This scaffold would also hold a reservoir of drugs, from which chemicals could trickle directly into the film and reach the bacteria.
Bed of Needles
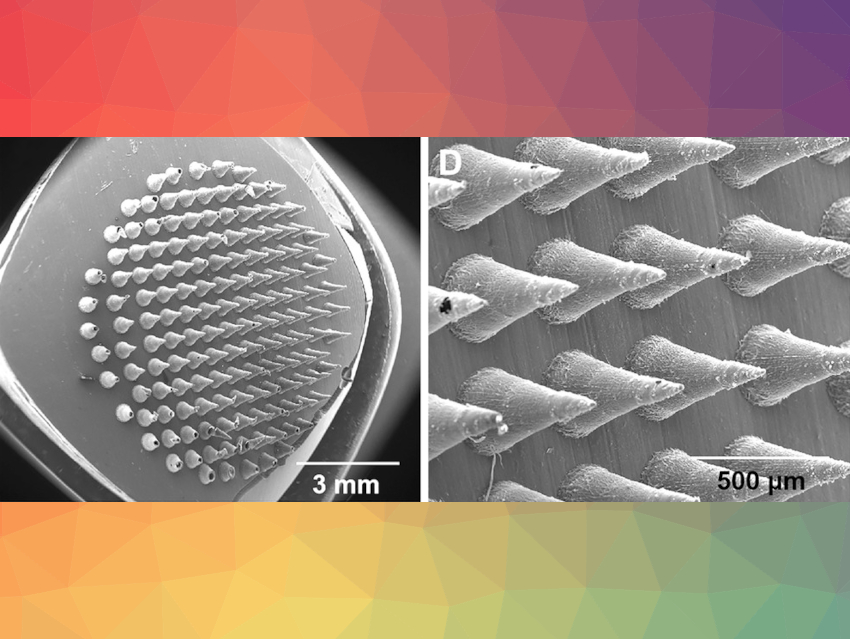
To enable penetration, one side of the patch is made like a miniaturized bed of nails (pictured), just the right size to prick holes in the surface of the biofilm. The other side consists of a fibrous cushion containing the antimicrobial reservoir. To make the microneedle side of the patch, the researchers first created a mold containing a dense array of cone-shaped hollows, half a millimeter in length. A solution of antimicrobial agents and water-soluble polyvinylpyrrolidone (PVP) was added to these hollows. After drying, the PVP microneedle bed was fully covered with a mat made of electrospun poly(ε-caprolactone) (PCL) fibers. These fibers have a core–sheath structure and can be filled with a solution of antimicrobial agents.
All the materials are fully degradable. “We chose PCL as raw material for the nanofiber mats as it is biodegradable, biocompatible, and has been used in the preparation of FDA-approved medical devices,” commented the team. The PVP used in the microneedle array is a hydrophilic binder material widely used in pharmaceutical preparations. As a water-soluble substance, it dissolves in contact with the wound.
Antimicrobial Cocktail
The researchers tested three established antimicrobial agents to produce their antimicrobial cocktail: silver ions, gallium(III) ions, and vancomycin. Silver interferes with bacterial respiratory pathways, while gallium(III) disrupts iron-dependent metabolic processes. Vancomycin, which often serves as a drug of “last resort”, is a narrow-spectrum glycopeptide antibiotic that acts against gram-positive bacteria such as Staphylococcus and Streptococcus, for example.
To test the biphasic patches, the researchers created a human wound model made of human skin samples retrieved during plastic surgery. On the model wound, they grew biofilms of methicillin-resistant Staphylococcus aureus (MRSA) cultures and observed what happened to the biofilms when they were covered with the biphasic patches.
Depositing the patches on the wounds destroyed the MRSA biofilms. Other films containing different bacterial cultures also vanished under the patches, which demonstrated that the patches could have wider applicability. The researchers recommend replacing the patches at least once for a successful treatment. Although they had designed the nanofibers to continuously release antimicrobial agents for days or even weeks, the PVP microneedles dissolved quickly in the aqueous wound environment. To renew the piercing effect, at least one patch change within two days was found to be the best option.
Synergy Improves Efficacy
The team observed that the three antimicrobial chemicals worked together in synergy. Patches containing only silver or gallium ions, or vancomycin alone, were also effective, but the combination of all three agents brought the most sustainable results, while only requiring one patch change. All other combinations or lone agents needed at least two replacements.
The researchers envision that these biphasic wound-dressing models may one day contribute to patient comfort. Neither high doses of antibiotics nor painful debridement would be necessary to treat chronic wounds. “This could improve the quality of wound care,” they say.
- Simultaneous Delivery of Multiple Antimicrobial Agents by Biphasic Scaffolds for Effective Treatment of Wound Biofilms,
Yajuan Su, Alec McCarthy, Shannon L. Wong, Ronald R. Hollins, Guangshun Wang, Jingwei Xie,
Adv. Healthcare Mater. 2021.
https://doi.org/10.1002/adhm.202100135