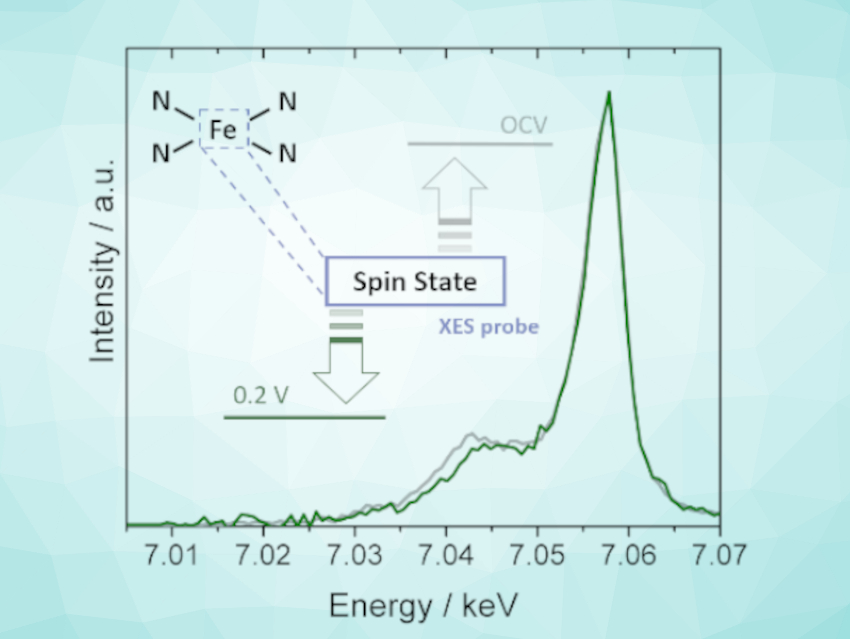
Inexpensive electrocatalysts are needed for the large-scale implementation of renewable energy conversion technologies such as fuel cells and electrolyzers. Materials featuring single iron atoms coordinated by nitrogen functional groups in a carbon matrix (Fe/N/C), for example, have potential as catalysts for the reduction of O2 or CO2. However, improving their electrocatalytic activity requires a better understanding of the properties of the active site.
Juan Herranz, Paul Scherrer Institute, Villigen, Switzerland, and colleagues have used X-ray emission spectroscopy (XES) to determine the electronic configuration of the active centers in two Fe/N/C catalysts. The catalysts were prepared via high-temperature pyrolysis of either an iron porphyrin on carbon or a mixture of a Zn(II)-based metal–organic framework (ZIF-8), phenanthroline, and iron acetate, respectively. The team collected XES spectra of both the catalysts and reference compounds with similar metal coordination environments and well-defined electronic properties.
The researchers were able to use the XES results to determine the average spin state of the catalysts. XES measurements carried out under electrochemical conditions revealed that the spin state can change with the potential. A comprehensive picture of the spin state changes undergone by Fe/N/C catalysts throughout the reaction could provide a better understanding of their catalytic mechanism.
- Potential-Induced Spin Changes in Fe/N/C Electrocatalysts Assessed by In Situ X-ray Emission Spectroscopy,
Viktoriia A. Saveleva, Kathrin Ebner, Lingmei Ni, Grigory Smolentsev, Daniel Klose, Andrea Zitolo, Elena Marelli, Jingkun Li, Marisa Medarde, Olga V. Safonova, Maarten Nachtegaal, Frédéric Jaouen, Ulrike I. Kramm, Thomas J. Schmidt, Juan Herranz,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202016951