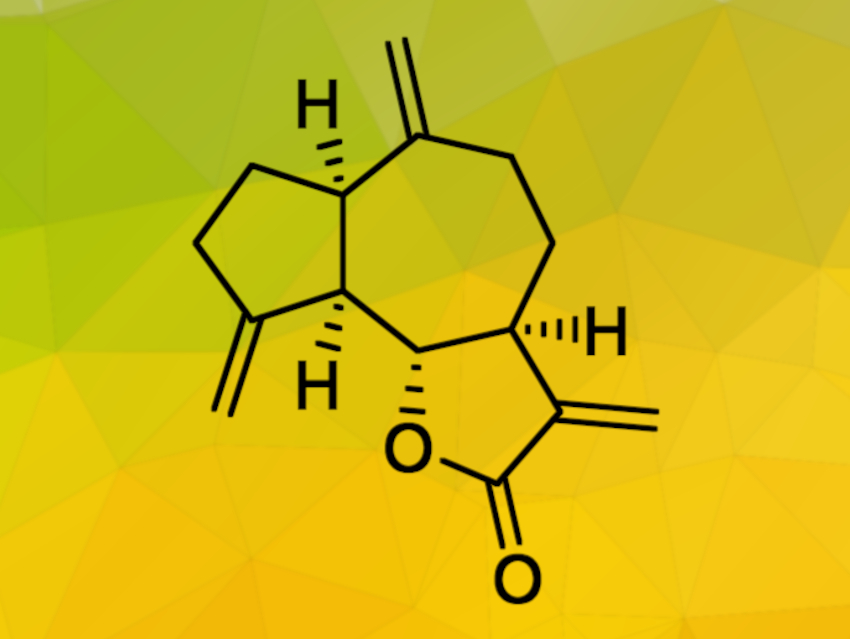
The sesquiterpenoid (−)‐dehydrocostus lactone (pictured above) has attracted research attention due to its potential anticancer activity. Although it can be isolated from various plants, the amount of pure natural product recovered in this way is very low. To allow for further studies of the biological properties of (−)‐dehydrocostus lactone, efficient synthetic pathways towards this natural product would be useful.
Peter Metz and colleagues, Technische Universität Dresden, Germany, have developed a high-yielding synthesis of (−)-dehydrocostus lactone using a domino metathesis reaction strategy. This synthesis can be achieved via two pathways: One of two different acyclic substrates, a dienyne (3) or an enediyne (2) (pictured below), was prepared in enantiopure form by asymmetric anti aldol reactions. Then, either a domino enediyne metathesis or a domino dienyne metathesis can be used to form hydroazulenes. These intermediates are functionalized using multiple hydroboration/oxidation reactions, establishing three out of four stereogenic centers of the target molecule in a single step.
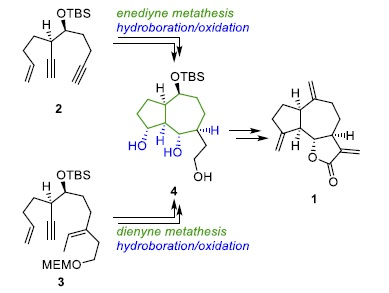
After a global oxidation of the polyol (4) formed this way, masking the γ-lactone moiety enabled the introduction of two exocyclic double bonds by double carbonyl olefination and thus, provided an improved path to (−)‐dehydrocostus lactone. The enediyne route has 17 linear steps with an overall yield of 12 %, while the dienyne route provides 17 % total yield over 21 steps.
- Asymmetric Total Synthesis of (−)‐Dehydrocostus Lactone by Domino Metathesis,
Felix Kaden, Susanne Nowotni, Franziska Höfner, Melanie Lorenz, André Barthel, Anne Jäger, Felix Hennersdorf, Jan J. Weigand, Peter Metz,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100681