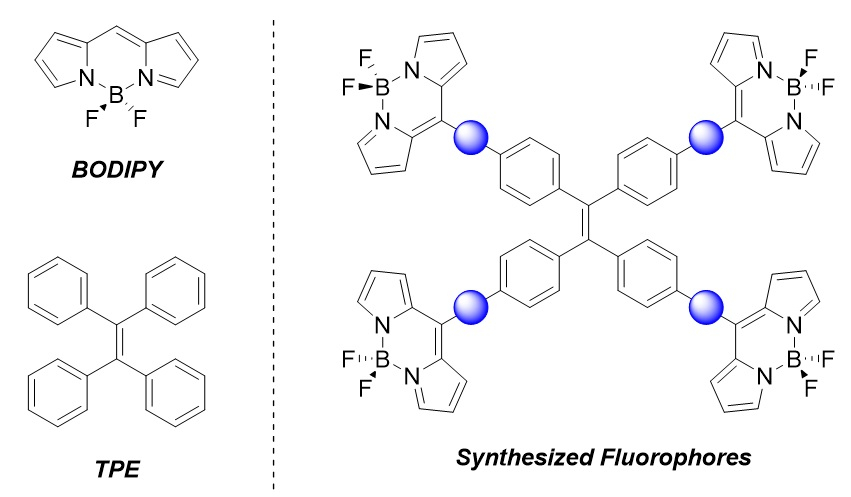
BODIPY (pictured below) consists of a BF2 group and a dipyrromethene unit. Dyes based on this structure are often used in applications such as protein tagging, pH sensing, and photodynamic therapy. Tetraphenylethylene (TPE, pictured below) and its derivatives show a phenomenon called aggregation-induced emission (AIE), i.e., they become emissive when they aggregate or crystallize. This class of molecule has applications ranging from organic light-emitting diodes (OLEDs) to biomedical imaging.
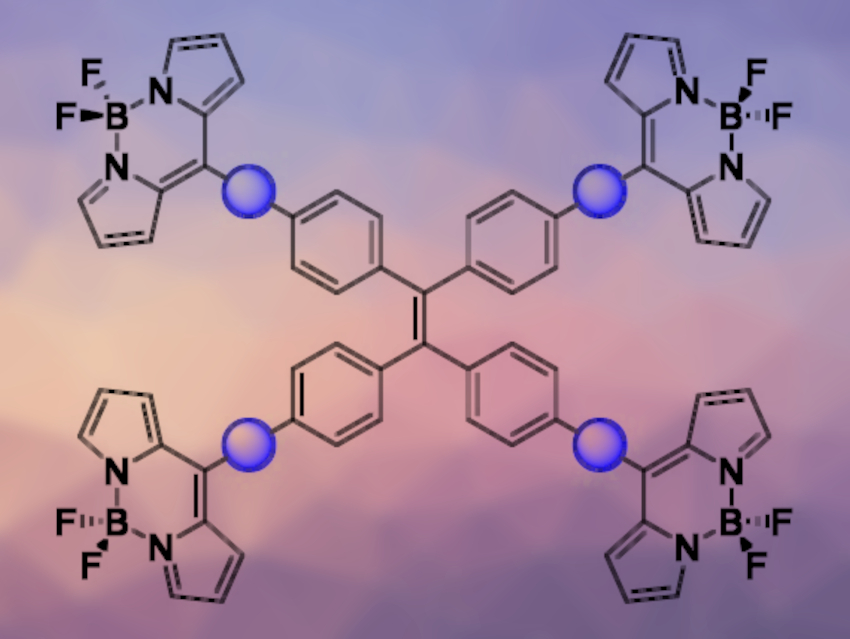
Mathias O. Senge, Trinity College Dublin, The University of Dublin, Ireland, and Technical University of Munich, Garching, Germany, and colleagues have synthesized tetra-BODIPY substituted TPEs (pictured below on the right). The team used palladium-catalyzed cross-coupling reactions, followed by an InCl3-mediated introduction of dipyrromethanes using pyrrole and a BF2 insertion, to install spacers and the BODIPY units onto the TPE backbone. The synthesized fluorophores vary in the spacer between the TPE and BODIPY cores (pictured as a blue circle): The team used either a phenylene or phenylethynyl spacer.
The phenylethynyl-spaced fluorophore showed a heightened AIE response in the form of aggregation-induced dual emission. According to the researchers, the dual emission is due to differing π–π stacking modes in the aggregated state. Both of the synthesized systems can produce singlet oxygen, which shows their potential for use as a photosensitizer. The fluorophores might be useful, e.g., in biomedical imaging, theragnostics, and light-harvesting arrays.
- Synthesis and Properties of BODIPY Appended Tetraphenylethylene Scaffolds as Photoactive Arrays,
Harry C. Sample, Ganapathi Emandi, Brendan Twamley, Nitika Grover, Bhavya Khurana, Vincent Sol, Mathias O. Senge,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100629