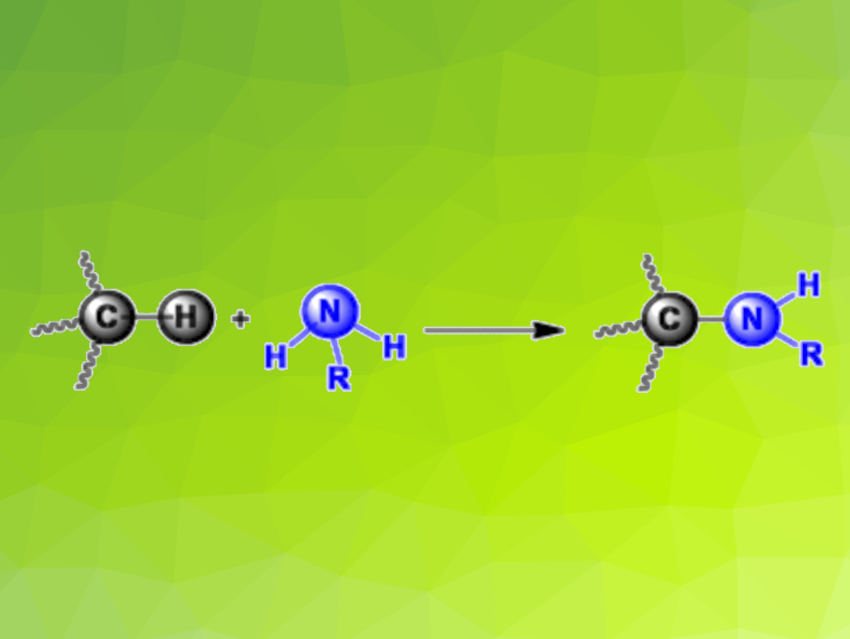
The carbon–hydrogen bonds in alkanes—particularly those at the ends of the molecules, where each carbon has three hydrogen atoms bound to it—are very hard to “crack” if you want to replace the hydrogen atoms with other atoms. Methane and ethane exclusively contain such tightly bound hydrogen atoms. Ana Caballero, Pedro J. Pérez,, Universidad de Huelva, Spain, John F. Hartwig, University of California, Berkeley, USA, and colleagues have discovered how they can break these bonds while forming new carbon–nitrogen bonds, i,e, in an amidation reaction.
Breaking C–H Bonds
If it were possible to easily break the C–H bonds in hydrocarbons, it would be possible to synthesize complex organic molecules, such as pharmaceuticals, much more conveniently and directly from petroleum. This strategy could also provide more pathways for recycling plastic waste. The formation of carbon–nitrogen bonds is of particular interest because these play an important role in natural products. For example, amide bonds link individual amino acids into proteins.
Although there has been some success in the functionalization of heavy hydrocarbons, even at the end positions, the particularly strong C–H bonds of light alkanes, especially methane, can hardly be split at all. The use of these primary components of natural gas as synthetic building blocks is especially desirable, as it would allow for the use of this often wasted side-product of oil extraction.
Dehydrogenative Amidation of Light Alkanes
The team has coupled amides to light alkanes with loss of a hydrogen atom. The products of these dehydrogenative amidations (pictured) are known as N-alkyl amides. The starting point for this approach was the amidation of C–H bonds in heavy alkanes with a copper-based catalyst and di-tert-butyl peroxide as an oxidizing agent, as developed several years ago by the Hartwig group. Variation of the catalyst then led to success. If the copper has phenanthroline-type ligands, it is possible to produce high yields in the reaction of ethane with benzamide—as well as a number of other amides—using benzene as a solvent. The reaction also worked when supercritical carbon dioxide—a more environmentally friendly option—was used as a solvent.
The reaction with ethane is an unusual C–N bond formation with a non-activated primary C–H bond. Propane, n-butane, and iso-butane gave similar results. In the light alkanes, reactivity correlates significantly more strongly with the dissociation energy of the C–H bonds than in higher alkanes. Even the toughest candidate, methane—amidation of methane has never previously been observed—could be coupled to the amide. Isotopic experiments were used to prove that methane reacts to form N-methylbenzamide.
- Copper‐Catalyzed Dehydrogenative Amidation of Light Alkanes,
M. Ángeles Fuentes, Riccardo Gava, Noam I. Saper, Erik A. Romero, Ana Caballero, John F. Hartwig, Pedro J. Pérez,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202104737