A substantial part of the plastics used in everyday life is produced by photopolymerization. A key component in this process is the photoinitiator (PI). PIs with low toxicity also have applications in the medical field, e.g., for dental fillings. Acylgermanes are promising compounds for use as PIs because of their excellent photoreactivity and low toxicity. However, a major drawback is their low solubility.
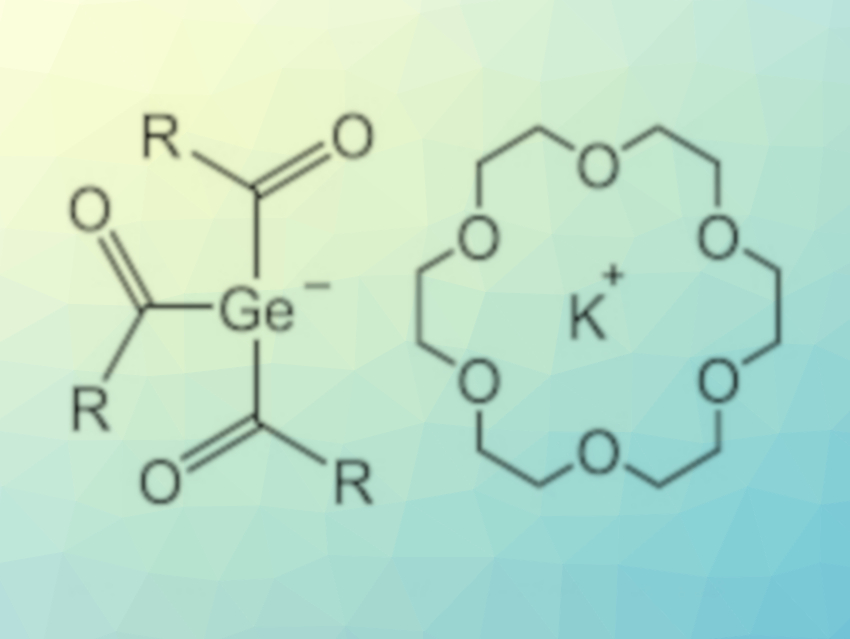
H. Bernhard Linden, Linden CMS GmbH, Weyhe, Germany, Michael Haas, Graz University of Technology, Austria, and colleagues have found a solution for this problem. They developed an easy-to-perform synthesis of triacylgermenolates (pictured in the center of the image below), which can act as building blocks for a variety of germanium-based PIs (pictured below on the right).
The team started from tetrakis(trimethylsilyl)germane, which was reacted with KOtBu in the presence of [18]-crown-6 to obtain the corresponding germanium anion. The anion was then reacted with three equivalents of an acid fluoride auch as o-toluoyl fluoride to obtain the desired triacylgermenolate. The method is restricted to derivatives containing ortho-substituted aromatic rings with electron-donating groups.
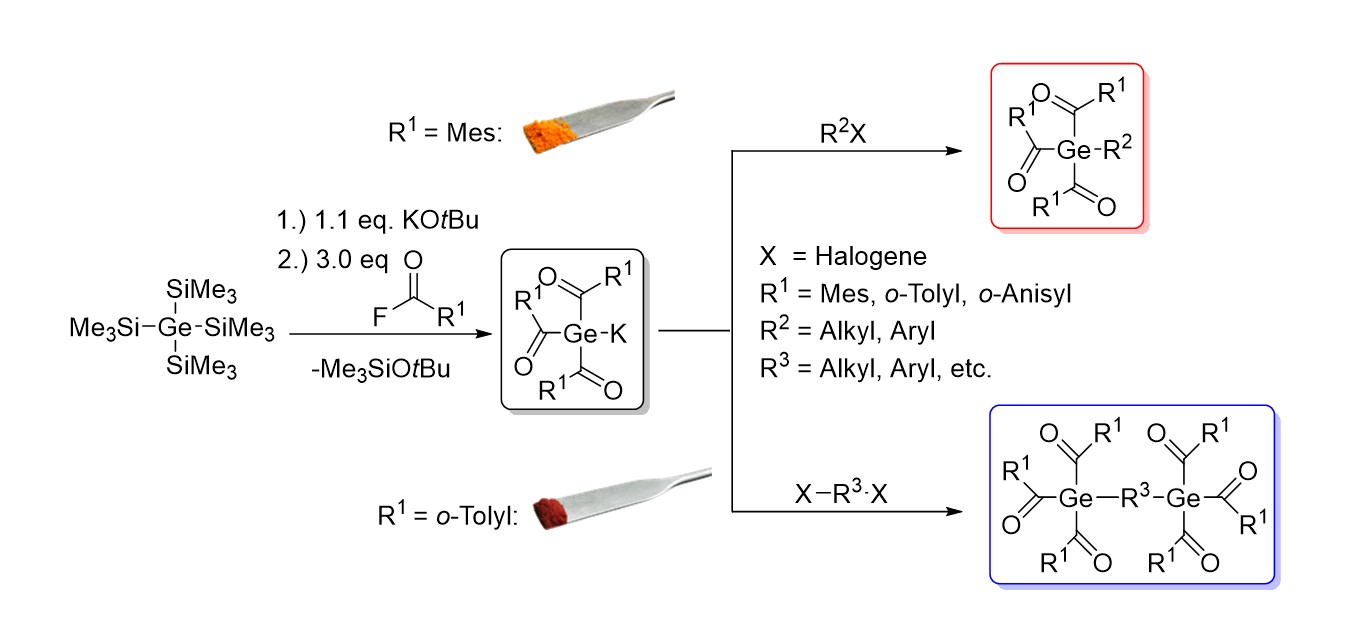
NMR spectroscopy, UV-Vis measurements, and LIFDI mass spectrometry were used to characterize the new germenolates. They can be reacted with a range of electrophiles, e.g., terephthaloyl chloride or bromoalkanes, to give new acylgermanes with useful solubility and/or light absorption properties.
- Synthesis, LIFDI Mass Spectrometry and Reactivity of Triacyl-Germenolates,
Manfred Drusgala, Mathias H. Linden, Andreas Knoechl, Ana Torvisco, Roland C. Fischer, H. Bernhard Linden, Michael Haas,
Eur. J. Inorg. Chem. 2021.
https://doi.org/10.1002/ejic.202100441