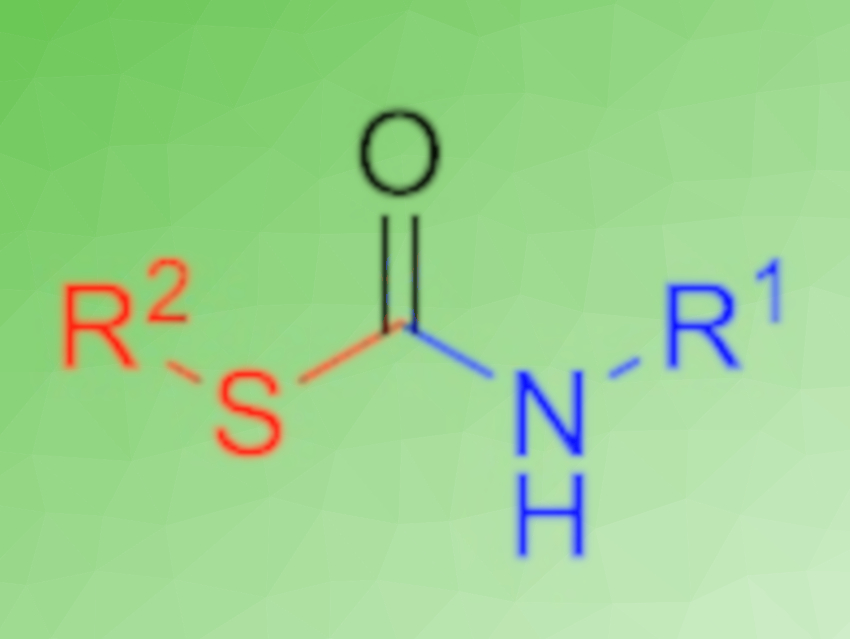
S-organyl thiocarbamates are often biologically active and can have applications, e.g., as pesticides and herbicides. Their synthesis typically relies on toxic compounds such as phosgene. To decrease the environmental impact and increase the efficiency of the synthesis of thiocarbamates, one-pot, multicomponent, low-waste protocols are desirable.
Michael A. R. Meier, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have developed a simple one-pot procedure to synthesize S-organyl thiocarbamates (pictured). The reaction uses N-formamides as starting materials, which are dehydrated using p-tosyl chloride to give isocyanides. The subsequent addition of a sulfoxide, without a purification step, converts these isocyanides to the desired thiocarbamates.
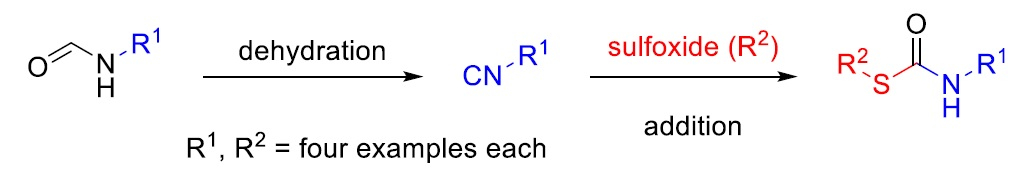
The protocol was used to synthesize a library of 16 S-thiocarbamates. The team, e.g., used the method to prepare norbornene-based thiocarbamates, which were then used in the synthesis of polymers via ring-opening metathesis polymerization (ROMP).
- One‐Pot Synthesis of Thiocarbamates,
Kevin A. Waibel, Dennis Barther, Triantafillia Malliaridou, Dafni Moatsou, Michael A. R. Meier,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100858