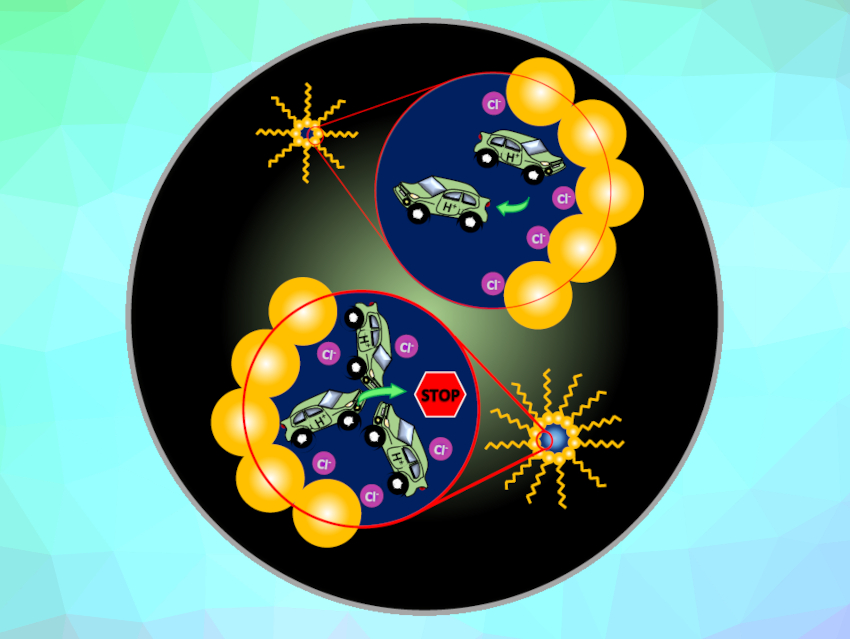
The passage of aqueous liquids across membranes is important for biological and artificial membrane surfaces, including fuel cells, for example. Water within confined environments creates a perturbed hydrogen-bonding network compared with the bulk water phase, which can change the transport of solvated protons.
Martina Havenith-Newen, University of Bochum, Germany, Teresa Head-Gordon, Lawrence Berkeley National Laboratory and University of California, Berkeley, CA, USA, and colleagues have investigated the proton-hopping mechanism in nanoconfined water pools containing hydrochloric acid, which were created by suspending a nonionic, alcohol-terminated surfactant in a nonpolar solvent to form reverse micelles. Using TeraHertz (THz) and dielectric relaxation (DR) spectroscopies, the team observed that proton transport within these nonionic reverse micelles is strongly dependent on the proton concentration and the size of the nanoconfined environment.
For small reverse micelles and/or low proton concentrations, the forward-hopping Grotthuss mechanism was dominant. In larger reverse micelles and/or at high proton concentrations, the proton became trapped and shuffled back and forth at the micelle interface. Proton transport was suppressed because the proton and its chloride counter ion accumulated at the micelle interface. As the micelle size and/or acid concentration increased, the interfacial water network induced a “traffic jam” that favored a localized oscillatory proton-hopping mechanism.
- Proton Traffic Jam: Effect of Nanoconfinement and Acid Concentration on Proton Hopping Mechanism,
Martina Havenith-Newen, Ellen M Adams, Teresa Head-Gordon, Hongxia Hao, Maximilian Rüttermann, Itai Leven, Hanna Wirtz,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202108766