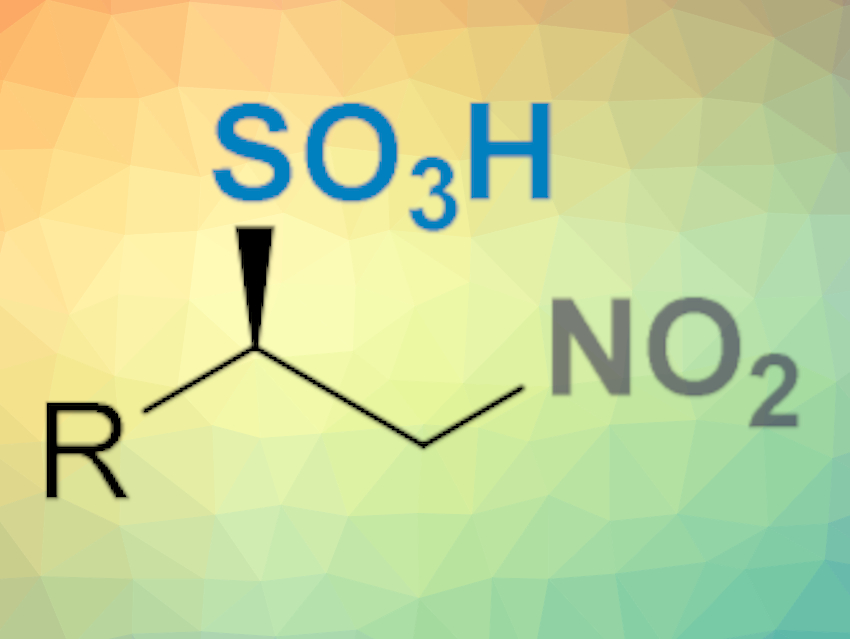
Chiral sulfonic acids and their derivatives are valuable chemicals in the pharmaceutical industry and in natural product chemistry. Examples include sulfonic acids and sulfamides with antibacterial activity, and β-amino sulfonic acids derived from taurine, a precursor of bile and an additive in energy drinks. Despite this, there are few methods to prepare these compounds in an enantioselective fashion.
Gonzalo Blay, José R. Pedro, Universitat de València, Spain, and colleagues have developed a simple, efficient, and enantioselective method for the synthesis of β-nitrosulfonic acids (pictured below). The procedure involves the reaction of cheap sodium bisulfite and β-substituted nitroalkenes in the presence of a chiral bifunctional thiourea as an organocatalyst in o-xylene/water at –20 °C.
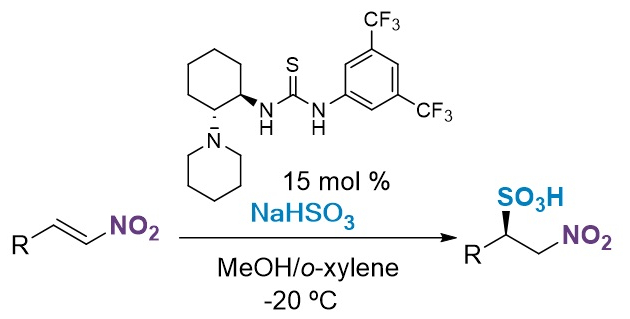
The large scope of the reaction was demonstrated with 17 substrates that provided the desired products in excellent yields (up to 99 %) and enantioselectivity (up to 96 % ee). The reaction tolerates a variety of aryl and alkyl substituents on the β-nitroalkenes.
- Enantioselective addition of sodium bisulfite to nitroalkenes. A convenient approach to chiral sulfonic acids,
Ehsan Sheikhi, Narjes Rezaei, Alvaro Castilla, Amparo Sanz-Marco, Carlos Vila, M. Carmen Muñoz, Jose R. Pedro, Gonzalo Blay,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202101064