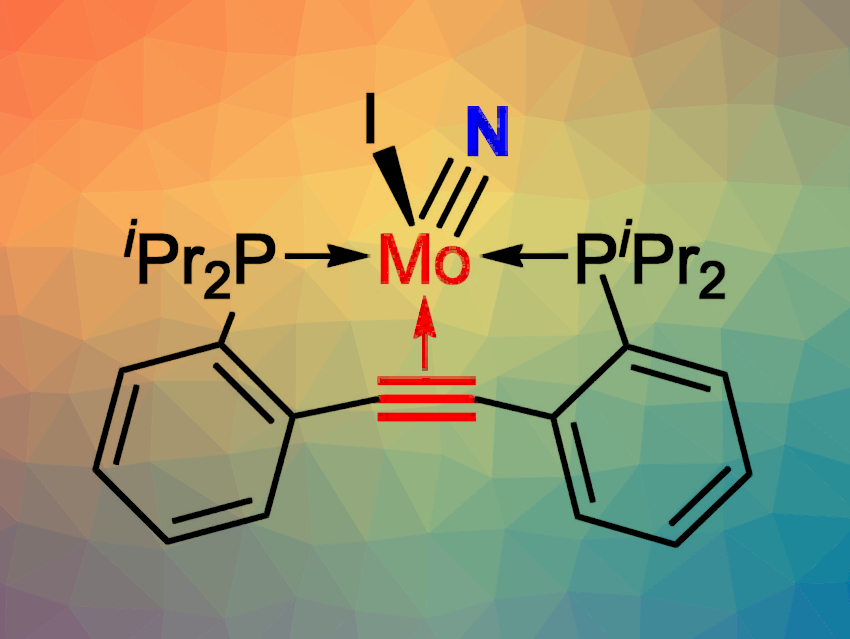
The cleavage of molecular N2 is often challenging, as six electrons are needed to reduce and split the N2 molecule. N2 cleavage reactions that have been reported to date have been carried out in the absence of olefins or alkynes, which are more easily reduced than gaseous N2.
Joachim Ballmann and colleagues, University of Heidelberg, Germany, have found that the cleavage of N2 can be accomplished in the presence of a coordinated alkyne. The team used a molybdenum complex together with a sodium/lead alloy as a reductant. To achieve the desired selectivity, the rotational freedom of the alkyne was restricted by embedding it within the backbone of a PCCP-type pincer ligand that coordinates through two P atoms and the alkyne group. A molybdenum nitrido complex (pictured) was obtained upon N2 splitting.
No side reactions of the alkyne were observed—not even during further N-functionalization of the molybdenum nitride. The finding that the strong N≡N bond can be cleaved in the presence of a weaker, but spatially constrained C≡C bond shows that alkyne reduction is not necessarily always preferred over N2 reduction
- Molybdenum‐Mediated N2‐Splitting and Functionalization in the Presence of a Coordinated Alkyne,
Hannah K. Wagner, Hubert Wadepohl, Joachim Ballmann,
Angew. Chem. Int. Ed. 2021.
https://doi.org/10.1002/anie.202111325