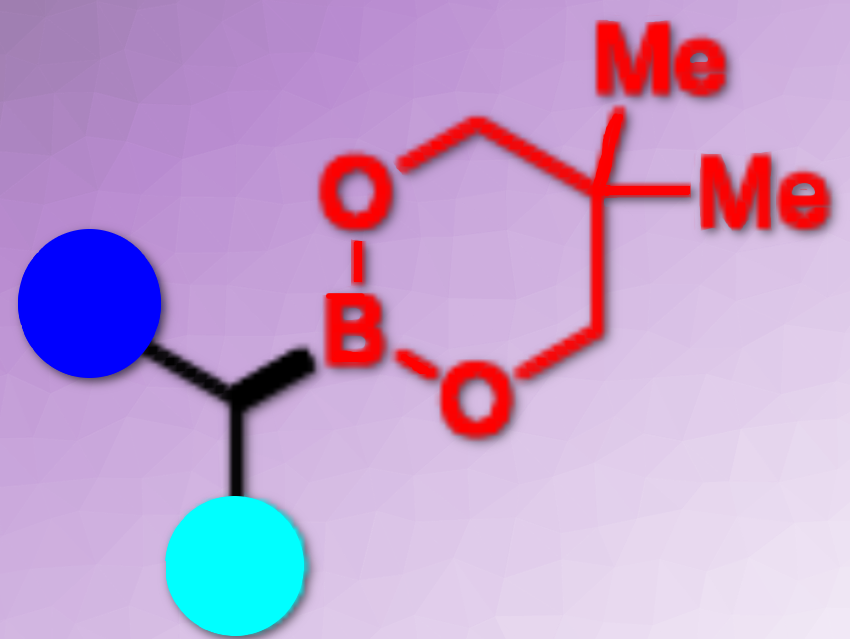
Alkylboronates are useful compounds in organic synthesis. Transition metal-free radical borylation protocols have emerged as an important tool to access alkylboronates. However, an esterification step is usually required. The development of such processes without the need for transesterification is desirable but challenging.
Todd B. Marder and colleagues, Julius-Maximilians-Universität Würzburg, Germany, have developed a practical and direct method for the production of versatile alkylboronic esters via transition metal-free borylation of primary and secondary alkyl sulfones. The team used bis(neopentyl glycolato) diboron (B2neop2) with a stoichiometric amount of base as a promoter for the borylation of alkyl sulfones (pictured below). Radical clock, radical trap experiments, and electron paramagnetic resonance (EPR) studies indicate that the borylation process involves radical intermediates.
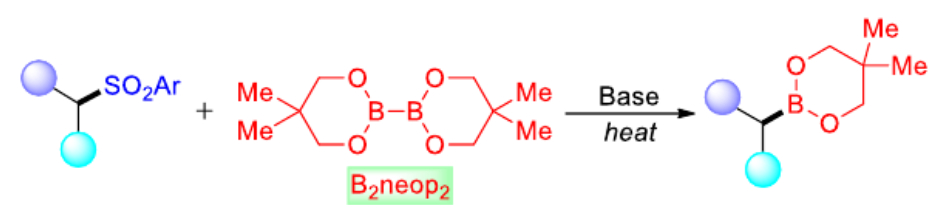
The reaction is tolerant to various functional groups and permits late-stage functionalization of complex molecules. An alkyl substrate bearing both sulfone and bromide groups showed good selectivity for borylation of the alkyl sulfone moiety. According to the researchers, these findings will prompt further development of desulfonative radical cross-coupling reactions.
- Base‐Mediated Radical Borylation of Alkyl Sulfones,
Mingming Huang, Jiefeng Hu, Ivo Krummenacher, Alexandra Friedrich, Holger Braunschweig, Stephen A. Westcott, Udo Radius, Todd B. Marder,
Chem. Eur. J. 2021.
https://doi.org/10.1002/chem.202103866