Organocatalysts can be useful in sustainable, “green” chemistry. Lewis acid organocatalysts, for example, can provide tunable reactivity and have a lowered environmental impact compared with more toxic, expensive, and/or sensitive metal-based Lewis acids. Triarylmethyl, or trityl, cations, in which a carbon atom bearing a positive charge is stabilized by three aromatic rings, can serve as Lewis acid organocatalysts. The stabilization is tunable by altering the substituents, allowing for the reactivity of the cations to match the demands of a particular reaction.
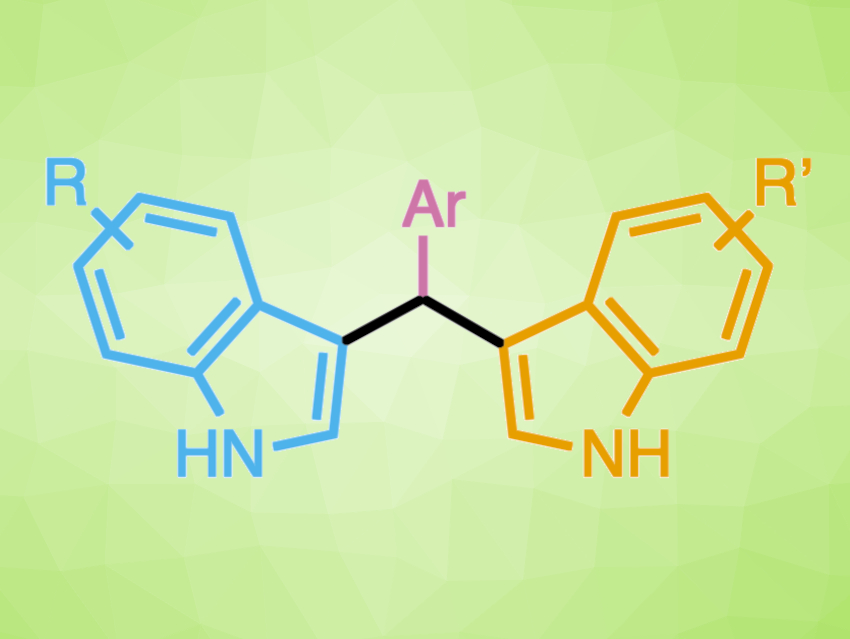
Cheyenne S. Brindle, Trinity College, Hartford, CT, USA, and colleagues have developed a method for the synthesis of bisindolylmethanes (pictured above) using trityl organocatalysts. Bisindolylmethanes contain two indole subunits. Most protocols available for the synthesis of bisindolylmethanes do not allow the two indole units to be varied independently. However, optimization of the organocatalyst allowed for single-addition selectivity in reactions with p-nitrophenyl imines (pictured in pink). The resulting intermediates can be isolated or used in situ in a one-pot, two-step reaction to generate unsymmetrical bisindolylmethanes (pictured below).
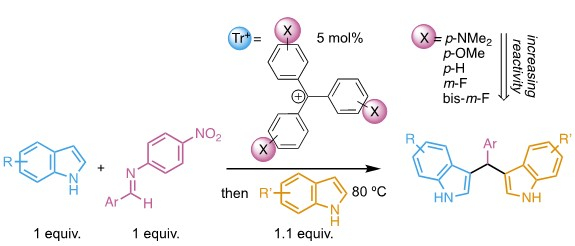
Overall, nonsymmetric bisindolylmethane products in which the two indole units can be varied independently are formed in high yields. The reaction minimizes waste and environmental impact by employing a one-to-one ratio of starting materials. According to the researchers, studies on the biological activity of the products are ongoing.
- Triarylmethyl Cation‐Catalyzed Three‐Component Coupling for the Synthesis of Unsymmetrical Bisindolylmethanes,
Cheyenne S Brindle, William J. Patterson, Kelly Lucas, Vanessa A. Jones, Zhenghua Chen, Kevin Bardelski, Melissa Guarino-Hotz,
Eur. J. Org. Chem. 2021.
https://doi.org/10.1002/ejoc.202100946