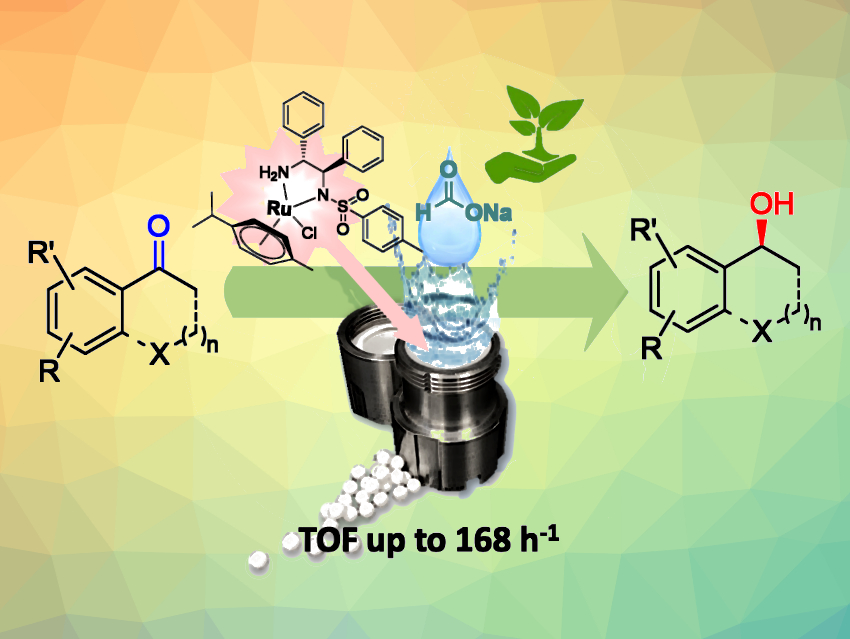
Optically pure alcohols are versatile building blocks, e.g., in pharmaceutical chemistry. Asymmetric catalytic methods, such as enantioselective transfer hydrogenations of ketones, are convenient processes for the enantioselective synthesis of alcohols. Mechanochemical activation could improve the sustainability and efficiency of these synthetic procedures.
Vanessza Judit Kolcsár and György Szőllősi, University of Szeged, Hungary, carried out asymmetric transfer hydrogenations of various aromatic ketones (pictured) using liquid-assisted grinding in aqueous media in a ball mill. In-situ formed ruthenium complexes of optically pure N-(4-toluenesulfonyl)-1,2-diphenylethane-1,2-diamines (TsDPEN) were used as catalysts, and sodium formate was used as a hydrogen donor.
Various ketones were transformed to the corresponding chiral alcohols with high conversions and enantioselectivities, requiring shorter reaction times than previously reported batch procedures. The mechanochemical method can be scaled up to obtain optically pure alcohols at a few-gram scale. The work shows that mechanochemical activation is an efficient way to carry out asymmetric transfer hydrogenations and obtain sufficient amounts of optically pure chiral building blocks for pharmaceutical use in a sustainable manner.
- Mechanochemical, Water‐Assisted Asymmetric Transfer Hydrogenation of Ketones Using Ruthenium Catalyst,
Vanessza Judit Kolcsár, György Szőllősi,
ChemCatChem 2021.
https://doi.org/10.1002/cctc.202101501