Carboxonium cations ([RR’C=OR”]+) are generally unstable species that are usually prepared and isolated at low temperatures. Species like protonated benzoic acid or diprotonated benzene dicarboxylic acids, for example, can be prepared under superacidic conditions. However, the behavior of tricarboxybenzenes in superacidic media had not been investigated so far.
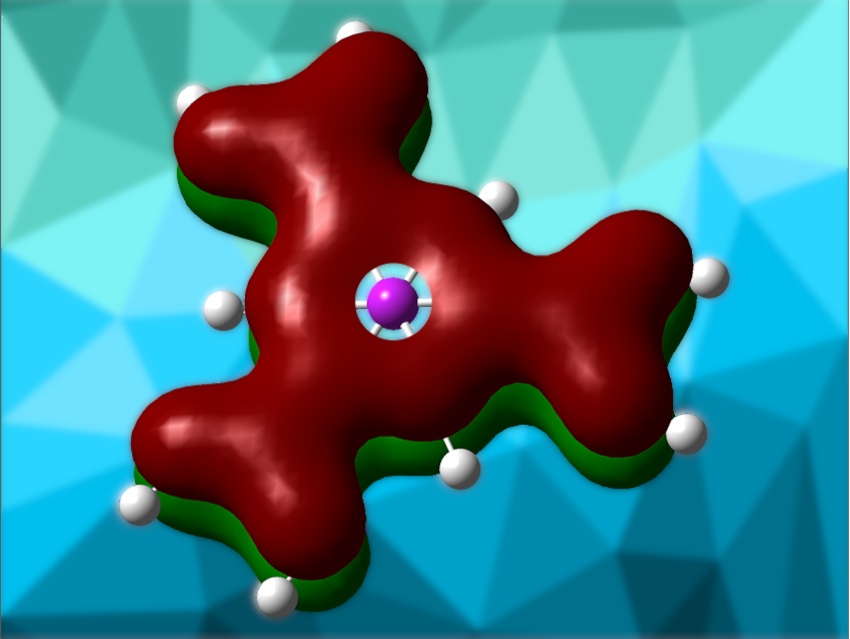
Andreas J. Kornath, Ludwig Maximilian University (LMU) Munich, Germany, and colleagues have investigated the (tri)protonation of the three isomers of tricarboxybenzene (example pictured below). The triprotonations of 1,3,5-tricarboxybenzene, 1,2,3-tricarboxybenzene, and 1,2,4-tricarboxybenzene were achieved. Superacidic systems like HF/BF3 or HF/SbF5 were used for the protonations, resulting in the formation of salts that are surprisingly stable—up to room temperature in some cases.
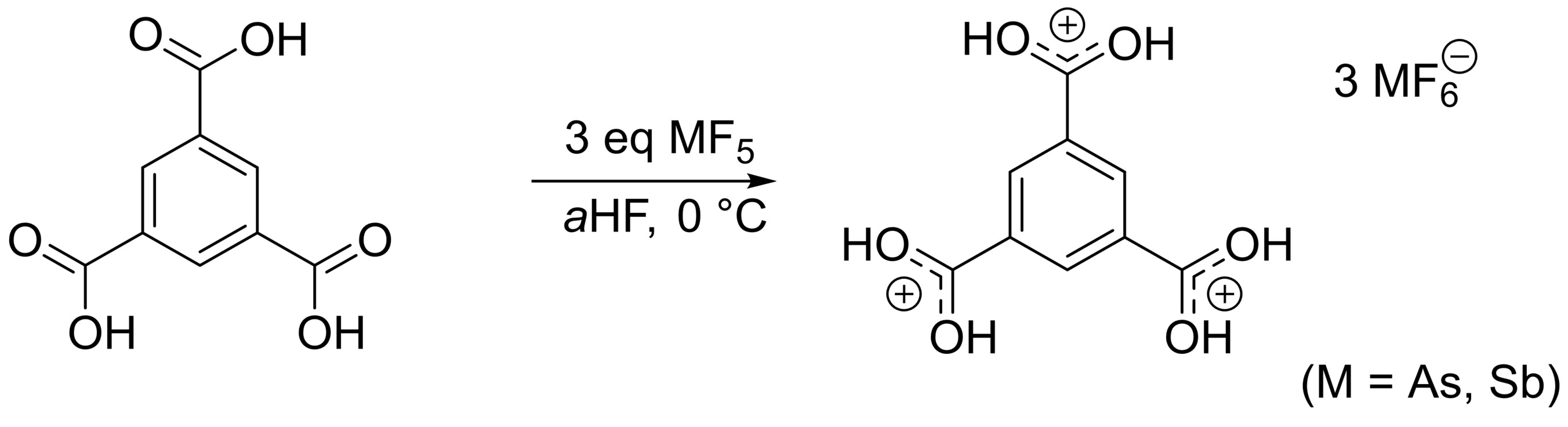
The team found that triprotonated 1,3,5-tricarboxybenzene (pictured) is the most stable isomer, with triprotonated 1,2,3-tricarboxybenzene second, and triprotonated 1,2,4-tricarboxybenzene the least stable. The researchers calculated the nucleus-independent chemical shift (NICS), which quantifies the ring current and is a measure of aromaticity. The protonation of the tricarboxybenzenes leads to an overall decrease in aromatic character, the amount of which depends on the substitution pattern.
- Third Time is a Charm – Protonating Tricarboxybenzenes,
Alexander Nitzer, Martin Regnat, Christoph Jessen, Andreas J. Kornath,
Eur. J. Org. Chem. 2022.
https://doi.org/10.1002/ejoc.202101488