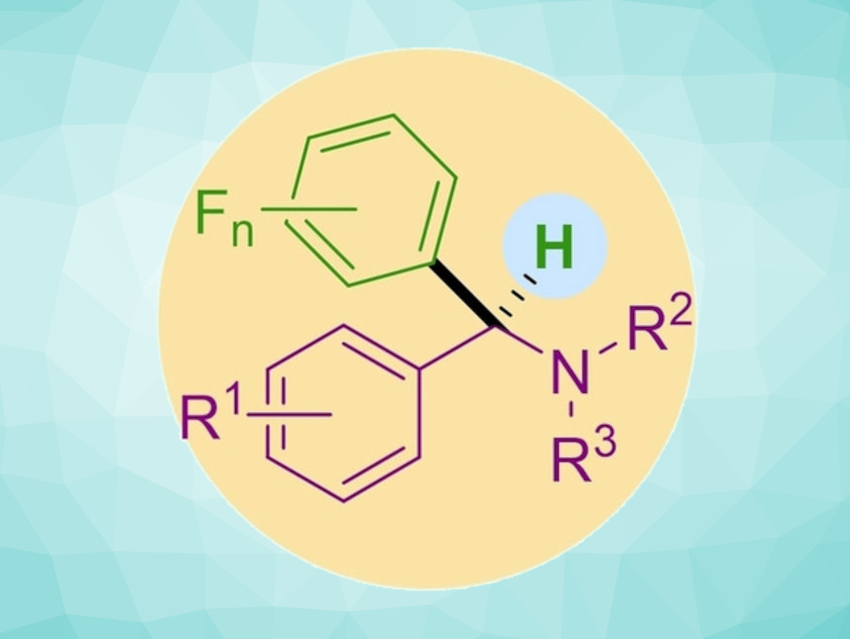
The efficient preparation of complex organic molecules starting from easily available, yet not very reactive substrates is an important challenge in organic chemistry. Amides, for example, are valuable precursors that can be converted to synthetically useful amines by deoxygenative C–C bond formation. However, the relative inertness of the amide C=O bond can make this difficult.
Xiaoming Wang, Shanghai Institute of Organic Chemistry and Hangzhou Institute for Advanced Study, University of the Chinese Academy of Sciences, Yu Lan, Zhengzhou University and Chongqing University, both China, and colleagues have developed a deoxygenative cross-coupling of aromatic amides with polyfluoroarenes (pictured below). The team used Sm/SmI2 to promote the reaction, AgBF4 as an additive. and tetrahydrofuran (THF) as the solvent. The reactions were performed at 60 °C.
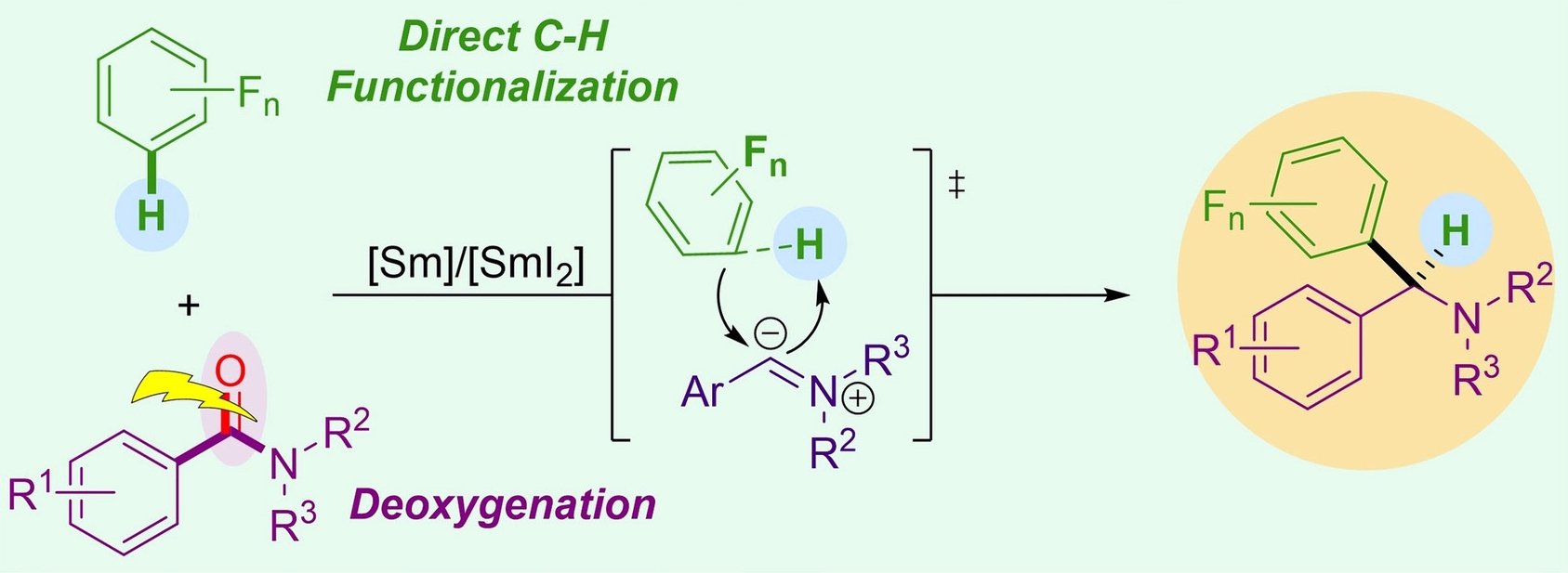
The aromatic amides act as readily available carbene precursors through an Sm/SmI2-mediated deoxygenation process. The insertion of the resulting carbene into the C–H bond of a polyfluoroarene gives a variety of α-polyfluoroaryl amines in a single step and moderate to good yields.
- Deoxygenative Cross‐Coupling of Aromatic Amides with Polyfluoroarenes,
Youliang He, Yuxiao Wang, Shi‐Jun Li, Yu Lan, Xiaoming Wang,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202115497