Low-valent silicon species, such as silylenes (R2Si:) or disilenes (R2Si=SiR2), have attracted considerable research attention. However, many of the reactions used to prepare such species use a metal-induced reduction of a silicon(IV) precursor to obtain the corresponding low-valent derivative. These harsh conditions led to low functional-group tolerance and allowed for only moderate complexity of the products.
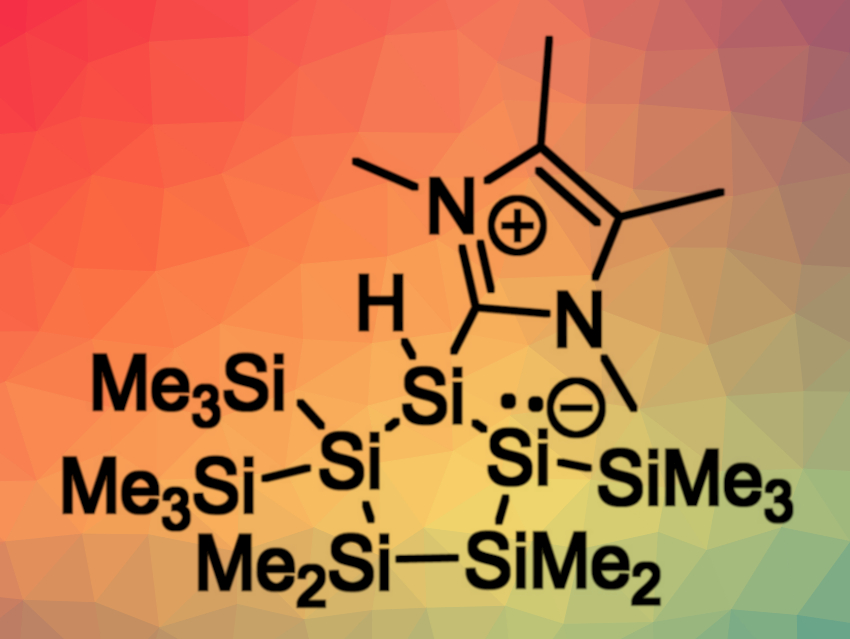
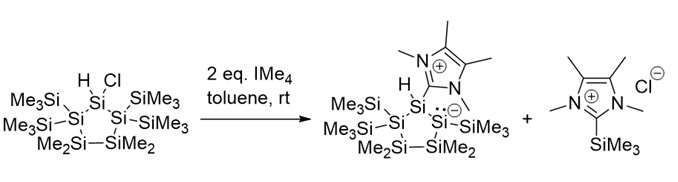
Anne-Marie Kelterer, Michael Haas, Graz University of Technology, Austria, Cameron Jones, Monash University, Australia, and colleagues have developed a metal-free synthesis approach towards disilenes. The team reacted a cyclic silicon(IV) precursor (pictured below) with two equivalents of an N-heterocyclic carbene (NHC, IMe4). They found that one NHC molecule reacts as a nucleophilic base to abstract a trimethylhalosilane, while the other equivalent of NHC serves as a Lewis base to stabilize the resulting endocyclic disilene. In the product, the disilene unit is incorporated in a cyclopolysilane framework (pictured above).
The disilene was characterized using NMR spectroscopy and X-ray crystallography. The product can undergo thermal or light-induced loss of the NHC, leading to a dimerization process that gives the corresponding dimer with an Si10 skeleton. The disilene can also react with transition-metal carbonyl complexes to give new tetracarbonyl iron-silyl complexes and pentacarbonyl molybdenum- and tungsten-silyl complexes.
- An NHC‐Mediated Metal‐Free Approach towards an NHC‐Coordinated Endocyclic Disilene,
Thomas Lainer, Deepak Dange, Michael Pillinger, Roland C. Fischer, Anne‐Marie Kelterer, Cameron Jones, Michael Haas,
ChemistryOpen 2022.
https://doi.org/10.1002/open.202100240