Radialenes are compounds that contain a ring of carbon atoms, each connected to a methylene (CH2) group outside of the ring by a double bond. The smallest radialene, [3]radialene, has a planar structure and unique optical and electrochemical properties. However, the synthesis of [3]radialene derivatives is very challenging due to the high strain in their three-membered carbon rings. A straightforward pathway to generate [3]radialenes would be a [1+1+1] cycloaddition, but this reaction had not yet been reported experimentally.
Deng-Yuan Li, Pei-Nian Liu, East China University of Science & Technology, Shanghai, Mengxi Liu, Xiaohui Qiu, National Center for Nanoscience and Technology and University of the Chinese Academy of Sciences, Beijing, Xing-Qiang Shi, Hebei University, Baoding, all China, and colleagues have performed the first [1+1+1] cycloaddition and atomically precise synthesis of an aza[3]radialene (pictured) from isocyanides on an Ag(111) surface. The team used 1,4-dichloro-2-isocyanobenzene (DCICB) as a precursor and dosed it onto an Ag(111) substrate held at 293 K under ultrahigh-vacuum conditions.
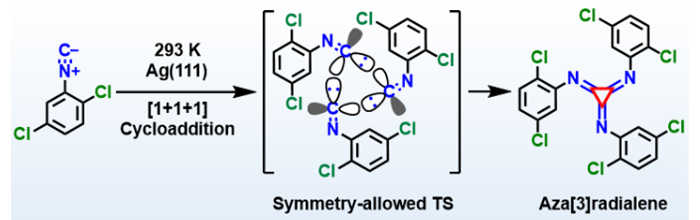
Using scanning tunneling microscopy (STM), non-contact atomic force microscopy (nc-AFM), time-of-flight secondary ion mass spectrometry (ToF-SIMS), and density functional theory (DFT) calculations, the team determined the structure of the produced aza[3]radialene. They found that the chlorine substituents of isocyanides guide the selectivity of the reaction via a sterically hindered transition state (TS, pictured). The work provides insight into the atomically precise synthesis of novel nanostructures.
- On‐Surface Synthesis of [3]Radialenes via [1+1+1] Cycloaddition,
Deng‐Yuan Li, Ying Wang, Xiao‐Yu Hou, Yin‐Ti Ren, Li‐Xia Kang, Fu‐Hua Xue, Ya‐Cheng Zhu, Jian‐Wei Liu, Mengxi Liu, Xing‐Qiang Shi, Xiaohui Qiu, Pei‐Nian Liu,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202117714
![On-Surface Synthesis of Aza[3]radialenes](https://www.chemistryviews.org/wp-content/uploads/legacy/common/images/thumbnails/source/17f18ebd28c.jpg)



