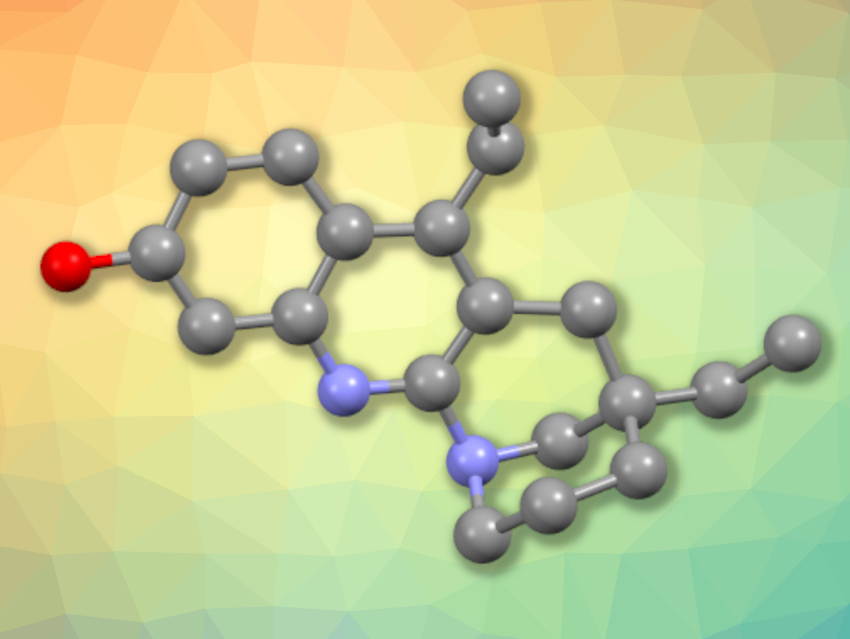
Eucophylline (structure pictured, hydrogen atoms omitted for clarity), a monoterpene indole alkaloid, was isolated from the bark of Malaysian Leuconotis eugenifolius. The compound has a tetrahydrobenzo[b][1,8]-naphthyridine skeleton.
Yannick Landais, University of Bordeaux, France, and colleagues have developed an efficient synthesis of (+)-eucophylline. The team used a sulfonyl-cyanation of a chiral cyclobutene and a nickel-boride mediated nitrile reduction/cyclization/ring-opening cascade to prepare a key six-membered ring lactam intermediate. This intermediate was first converted to a highly strained bridgehead amide and then to the tetracyclic core of eucophylline. The vinyl unit of the desired product was then installed via a Suzuki coupling.
Eucophylline was obtained in 17 steps starting from 1,1-dibromobutene, with an overall yield of 5.9 % and 98 % enantiomeric excess. In this synthesis, no protecting group was necessary and only nine steps required purification through column chromatography. X-ray diffraction studies and circular dichroism analyses confirmed the successful synthesis of the natural enantiomer.
- Enantioselective Total Synthesis of (+)‐Eucophylline,
Iman Traboulsi, Nitin S. Dange, Vincent Pirenne, Frédéric Robert, Yannick Landais,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202200088