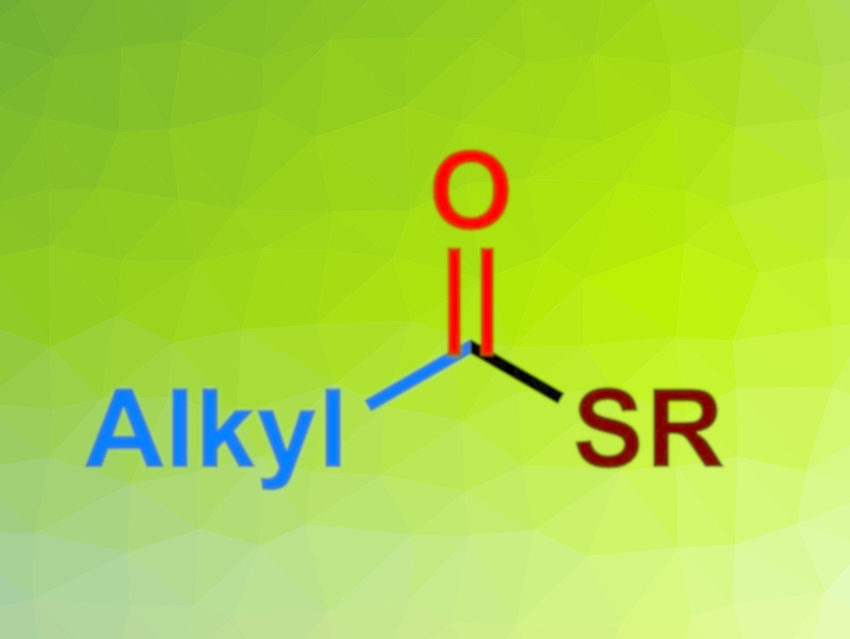
Molecules containing thioester functional groups are important, e.g., in the pharmaceutical and agrochemical fields. In addition, they can be used as convenient acyl donors in organic synthesis. However, traditional methods for the synthesis of thioesters can have drawbacks such as a need for harsh reaction conditions, odorous materials, or stoichiometric oxidants.
Yahui Li, Anhui Agricultural University, Hefei, China, and colleagues have developed a general procedure to synthesize alkyl thioesters via a Cu/Co co-catalyzed carbonylation of alkyl iodides using alkyl thioesters as reagents (pictured below). The team used S-alkyl butanethioates as odorless thiol surrogates. The copper N-heterocyclic carbene (NHC) complex 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene]copper(I) chloride was used as the Cu catalyst and the cobalt complex salcomine as the Co catalyst. KOtBu was chosen as the base and a xylene/anisole mixture as the solvent. The reactions were performed under 40 bar CO at 80 °C.
![]()
The reaction displays a good functional group tolerance. The desired thioesters were obtained in moderate to good yields.
- Co/Cu Co‐Catalyzed Carbonylation of Alkyl Iodides and Thioesters,
Lili Wang, Xia Wu, Qingqiang Tian, Yahui Li,
ChemistrySelect 2022.
https://doi.org/10.1002/slct.202103503