For a Japanese gourmet, winter is the most wonderful time of the year, because that is when the pufferfish, or fugu (its Japanese name) tastes best. Unfortunately, it’s also when it is at its most poisonous. The fugu poison, tetrodotoxin, falls very near the top of the international hit parade of poisons [1]. Swallowing even half a milligram transforms this culinary delicacy into an “executioner’s last meal”. You will probably have already guessed that such a poisonous species must also be a brilliant chemist.
1 The Fugu
The fugu, with its slimy skin, squat figure, rubbery lips, and bugged-out eyes would not be likely to win a beauty contest anywhere in the fish world (see Fig. 1). Tiny fins cause it to be such a slow swimmer that it is one of those species that has had to develop a defense strategy other than flight: so with lightning speed it can swallow enormous amounts of water, and in the process inflate itself (or “puff up”) quite dramatically! As it does so, the few remaining scales are shed from its body, and what was once a rather inconspicuous, ordinary fish becomes a huge, prickly, and frightening sphere — hence the English name “pufferfish” (or “blowfish”). In the face of a monstrosity like this, the erstwhile predator makes one exploratory pass around the remarkable fish, and can’t find any way either to gulp the fugu down whole, or even bite off a single tender morsel. (Have you ever tried biting into a whole melon?)

Figure 1. An example of a fugu.
The fugu has yet another defensive weapon in its arsenal: it is highly toxic! There is enough poison in a single full-grown fugu to paralyze within minutes 30 human beings, sending all of them in the course of a few hours straight to the hereafter. Other members of its own species are immune to the poison, but to fish in general it’s deadly, just as it is to other higher forms of life.
Both of these inherently highly effective defense mechanisms — the fish’s ability to inflate itself into a giant sphere, and the presence of a lethal poison — have helped protect the fugu from many attacks by humans, but evolution unfortunately made one big mistake: just as with the eel, which has the advantage for people wishing to eat it of containing only a single large bone, fugu flesh again is essentially free of bones, and it has a delicious flavor. Fear of the fugu’s deadly poison seems only to enhance its attraction, however, and in Japan over 10,000 tons of the fish are consumed annually.
The best fugu comes from Shimonoseki, in the straits between the Japanese islands of Kyushu and Honshu. From here, Tokyo and the other Japanese metropolitan areas receive fresh shipments daily. Due to high demand, young fish are caught in late spring and raised in large floating fish cages in the ocean.
Of the roughly 15 fugu species, fugu rubripes rubripes is the most highly prized. Because of its characteristic skin pattern, the Japanese refer to it as torafugu (tiger fugu). The fish is often eaten raw, so it needs to be extremely fresh. When fugu are to be transported live, their jaws are sewn shut to prevent them from injuring each other with their sharp teeth. Good fugu restaurants (ryotei) count on daily shipments, and they usually display their wares in an aquarium in the middle of the dining room.
2 The Fugu Poison
The most effective of the poisons from the plant world — such as morphine, atropine, strychnine, etc. — were in most cases already isolated in pure, crystalline form in the 19th century, but despite many attempts, preparation of a sample of pure tetrodotoxin was not achieved until the early 1950s. This was a consequence of the fact that plant poisons belong to the alkaloid class of substances, which are only slightly soluble in water but highly soluble in organic solvents. Tried and true separation techniques that were highly effective with alkaloids failed completely with tetrodotoxin, however.
2.1 Preparation in Pure Form
If someday you should get the urge to isolate a couple of grams of tetrodotoxin, the following “do it yourself” method [2] has been found to be reliable:
First, go to the Shimonoseki fish market, preferably in March, and buy 1000 kg of fugu roe. That will require about 5000 of the fish. It would probably be best to preserve the roe in formalin — on site — and then take it home in casks. The chopped up roe should be heated in water in your home laboratory for 20 minutes at 80–90 °C. Flocculated protein is then filtered out, and the aqueous solution carefully evaporated.
After purification with activated charcoal, you should extract with a methanol/water mixture that has been slightly acidified with acetic acid, after which you make the mixture slightly alkaline again with aqueous ammonia. Product that precipitates is dissolved in ethanol/acetic acid, and this is in turn treated with ether, causing the accumulation of tetrodotoxin in crystalline form.
Careful work should lead to ca. 8–9 g of tetrodotoxin, which turns brown above about 220 °C. Crystalline tetrodotoxin is insoluble in water as well as in organic solvents, but highly soluble in acidified water or methanol.
The molecular formula of the compound is C11H17O8N3, and it has an optical rotation aD = – 8.1°.
2.2 Establishing the Structure
Following the successful preparation of a pure sample of tetrodotoxin in the 1950s, a race broke out to be the first to determine its structure. This ended in a collective, harmonious finish involving no less than four research groups: those of T. Goto, H. S. Mosher, K. Tsuda and R. B. Woodward. In April 1964 all four groups reported on their successful tetrodotoxin structure determinations [3–6] at an IUPAC Symposium on the Chemistry of Natural Products held in Kyoto, Japan.
Strictly speaking, Mosher’s group did not actually study poison from the fugu, but focused instead on an isolation and structure determination of tarichatoxin, a poison from the California newt taricha torosa. In the course of these studies, however, Mosher recognized the latter’s close pharmacological similarity to tetrodotoxin, and comparison with a sample of authentic tetrodotoxin established — astonishingly — that poison from the fugu and that from the salamander class to which the newt belonged were identical. Since human beings don’t generally eat newts, the newts would normally not present a danger to us. There are exceptions, however: one young man did eat a California newt in response to a dare. He died a few hours later [7].
Determining the structure of tetrodotoxin proved to be a difficult undertaking. Here we single out for consideration only one tiny facet of the project. The molecule’s general structure was in fact known already from degradation reactions, but a precise structure determination nowadays requires finding some derivative that crystallizes well, and will then be available for X-ray analysis. Jack Gougoutas, a Woodward postdoc with especially good luck in this respect, discovered that when tetrodotoxin is treated with hydrochloric acid in acetone/methanol, beautiful crystals form. The resulting compound 1 was named — in honor of its discoverer — “Gougoutas hydrochloride” (see Fig. 2).
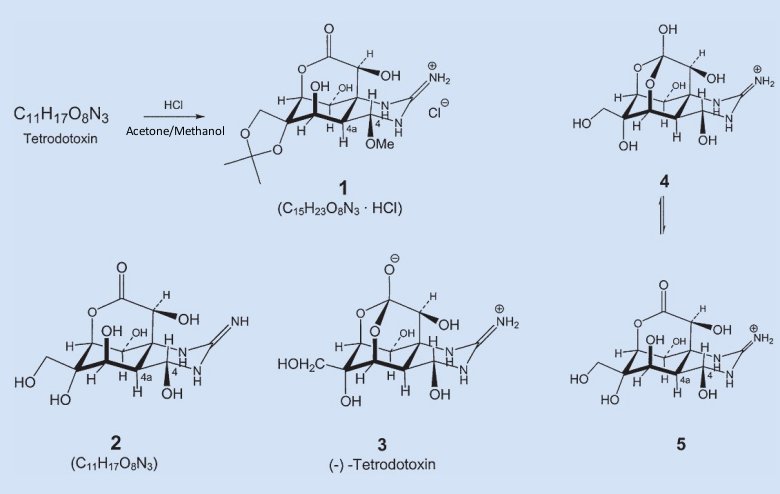
Figure 2. Tetrodotoxin (3).
Understandably, the reader who has not been trained in organic chemistry is bound to simply throw up his (or her) hands at the sight of such a bewildering molecular monstrosity, with its tangle of carbocyclic and heterocyclic rings.
The next systematic step in the project consisted of working backward from the structure of derivative 1 to the parent compound, tetrodotoxin. Ordinarily that shouldn’t be difficult, since we know the molecular formulas for both starting material and product, and gougoutas hydrochloride is made by treating tetrodotoxin with hydrochloric acid/methanol/acetone under very mild conditions.
Comparing the two molecular formulas, one concludes that tetrodotoxin must have reacted with one mole of acetone (CH3)2C=O and one mole of methanol CH3OH, while eliminating two moles of water. The rest ought now to be child’s play: in the presence of compounds (diols) with two –OH groups, and under acid catalysis, acetone forms cyclic acetals (1,3-dioxolanes) — protective groups highly prized by sugar chemists.
One can’t help noticing immediately that gougoutas hydrochloride contains an isopropylidene group (CH3)2C bonded to two oxygen atoms, and this must in turn have come from the acetone. The only other methyl group in the compound is attached via an oxygen atom at C-4, so the latter must originally have been an OH group. Finally, the only thing left is elimination of HCl, where the proton in question presumably comes from the protonated nitrogen of the guanidine group. These considerations lead us to structure 2 for tetrodotoxin.
Unfortunately, it’s wrong! Tetrodotoxin can’t be a lactone, because the highly characteristic band for a C=O double bond is absent from tetrodotoxin’s infrared spectrum. But the IR spectrum does provide a critical clue to the structure. Vibrations representing the protonated guanidinium group in gougoutas hydrochloride 1 are identical to ones also found in tetrodotoxin. In other words, tetrodotoxin must contain this same protonated guanidinium group.
More studies and thought were required before the entire structural puzzle fell into place, however: tetrodotoxin in fact corresponds to structure 3, and is therefore a 4,10,11,12-tetrahydroxy-12-(hydroxymethyl)-2-immonio-octahydro-5,9:7,10-dimethano[1,3]dioxocino[6,5-d]pyrimidin-7-olate.
Buried behind this complicated systematic name is a sensation!
Tetrodotoxin contains a functional group that was previously completely unknown: a hemilactal anion. Just imagine: a new natural product, containing a functional group that until then had never been seen in nature, and never even been prepared in a laboratory!
But tetrodotoxin 3 had yet another surprise up its sleeve: in acid solution there exists an equilibrium between the protonated hemilactal form 4 on the guanidine residue [8], and the hydroxylactone form 5 [9].
There has of course been no shortage of attempts to synthesize tetrodotoxin, as a means of gaining access to flexible derivatives. Kishi et al. actually succeeded in carrying out a total synthesis of racemic (±)-tetrodotoxin in 1972 [10].
Despite many tries [11 – 13], there still has been no stereoselective total synthesis of the natural (–)-enantiomer.
3 The Fugu Feast: a Culinary Thrill for Both Palate and Nerves
Let us take the opportunity also to enjoy tetrodotoxin sensually in the form of a festive fugu banquet. For the Japanese, a fugu dinner is always a very special occasion. One doesn’t simply walk into an upscale fugu restaurant (Fig. 3) as if it were a sushi bar, though. Instead, one reserves a table long in advance, and takes pains to organize the guest list carefully. The best plan would be for you to arrange to be one of the invitees, because — depending on quality and atmosphere — a fugu meal costs per person in the range of 100–300 Euros (ca. $150–450).
We begin our fugu banquet by selecting a magnificent specimen from the aquarium in the fugu ryotei. While we are being led to our table, the chosen fish will disappear into the kitchen, where the fugu chef is waiting for it. Hopefully tonight he’ll have a steady hand, because our later well-being will depend upon his skill.
At least there’s no need to worry about his credentials, however: they are first-rate. Since 1949 it has been required that every fugu chef undergo a three-year training period in cutting up and preparing the fish, culminating in an examination administered by the Japanese koseisho (Ministry of Health and Social Affairs). Above all, proper gutting is crucial, since the poison is not distributed evenly throughout the fish. Instead, it is found concentrated in the fish’s liver, intestine, roe, and skin. The tender flesh of interest to us is decidedly less poisonous.
Apart from the practical matter of cutting up the fish, the government examination also covers the ability to reliably recognize the various fugu species, as well as proper preparation and presentation of the dish on a platter.
The examination is a challenging one: only 30 % of candidates pass, being then allowed to graduate to the severest form of test-anxiety imaginable: eating fugu they themselves have prepared. But we can be perfectly confident and relax, knowing that of course our fugu chef has properly eviscerated the fish, completely removing liver, roe, and intestines, and washing everything thoroughly. So let the culinary thrills begin!

Figure 3. A fugu restaurant (fugu ryotei) is distinguishable by the pufferfish lantern hanging over the door and a fish painted over the entrance.
Hirezake
As a first course, hirezake, we are served the tiny fins: grilled, over which has then been poured hot sake. Hirezake turns out to be a bold appetizer, with a strong, fishy taste. Thanks to the hot sake we are quickly warmed up, the alcohol begins to take effect, and a cheerful atmosphere descends over the table.
Fugusashi
In the second course, fugusashi, raw fish is cut in paper-thin, almost transparent slices. These will have been carefully arranged on a platter, generally in the shape of a chrysanthemum, the flower of death (!). A great deal of effort is expended on the presentation: especially in Japan, the eyes are very much a part of a feast. Apart from aesthetic display, in Japanese cuisine not only the flavor but also the texture of the various components is important. Good fugusashi tastes shiko-shiko, a cross between tender and crispy.
Individual tender slices of fish are grasped with chopsticks, and then briefly immersed prior to eating in a dip you would almost certainly have prepared yourself by squeezing the juice from half of a wild orange (sudashi, Poncirus trifoliata) over a mixture of green onions, grated radish, and finely chopped bell pepper.
Fugushiri
At last, in the third course, fugushiri, you have a chance to actually become full! At the table there is prepared a fish stew with noodles, mushroom, carrots, Chinese cabbage, tofu and — of course — fugu.
An evening spent over a fugu meal with all its trappings is quite an exciting experience, and certainly memorable. From the standpoint of flavor, however, one will generally come away somewhat disappointed, if only because of expectations that were too high. In any case, though, few Europeans would probably go so far as the artist and gourmet Kitaoji Rosanjin, who asserted that “he who declines the offer of a piece of fugu for fear of dying is a truly pathetic individual”.
The Chemist’s Fear of the Fugu — Part 2
What to do if one’s fugu banquet seems not to sit well?
The Chemist’s Fear of the Fugu — Part 3
Klaus Roth considers the poison tetrodotoxin from the fugu’s perspecti
References
[1] H.-J. Quadbeck-Seeger, Chemie-Rekorde, Wiley-VCH, Weinheim, 1999.
[2] K. Tsuda, Naturwissenschaften, 1966, 53, 171. DOI: 10.1007/BF00631468
[3] T. Goto, Y. Kishi, S. Takahashi, Y. Hirata, Tetrahedron, 1965, 21, 2059. DOI: 10.1016/S0040-4020(01)98344-9
[4] H.S. Mosher, F. A. Fuhrmann, H. D. Buchwald, and H.G. Fischer, Science, 1964, 144, 1100-1110. DOI: 10.1126/science.144.3622.1100
[5] K. Tsuda, Chem. Pharm. Bull. Japan, 1964, 12, 634–642.
[6] R. B. Woodward, Pure Appl. Chem. 1964, 9, 49. DOI: 10.1351/pac196409010049
[7] S. S. G. Bradley, L. J. Kilka, J. Amer. Med. Ass. 1981, 246, 247. DOI: 10.1001/jama.1981.03320030039026
[8] A. Furusaki, Y. Tomiie, I. Nitta, Bull. Chem. Soc. Jpn 1970, 43, 3332.
[9] H.S. Mosher, Ann. N.Y. Acad. Sci. 1986, 479, 32.
[10] Y. Kishi, T. Fukuyama, M. Aratani, F. Nakatsubo, T. Goto, S. Inoue, H. Tanino, S. Sugiura, H. Kakoi, J. Am. Chem. Soc. 1972, 94, 9219. DOI: 10.1021/ja00781a039
[11] J. F. W. Keana et al., J. Org. Chem. 1983, 48, 3627, and literature cited. DOI: 10.1021/jo00169a002
[12] M. Funabashi, H. Wakai, K. Sato, J. Yoshimura, J. Chem. Soc., Perkin I, 1980, 14, and literature cited. DOI: 10.1039/P19800000014
[13] M. Isobe, T. Nishikawa, N. Fukami, T. Goto, Pure Appl. Chem. 1987, 59, 399. DOI: 10.1351/pac198759030399
The article has been published in German in:
and was translated by W. E. Russey.
Other articles by Klaus Roth published by ChemistryViews magazine:
- In Espresso — A Three-Step Preparation
Klaus Roth proves that no culinary masterpiece can be achieved without a basic knowledge of chemistry
DOI: 10.1002/chemv.201000003 - In Chocolate — The Noblest Polymorphism
Klaus Roth proves only chemistry is able to produce such a celestial pleasure
DOI: 10.1002/chemv.201000021 - In Sparkling Wine, Champagne & Co
Klaus Roth shows that only chemistry can be this tingling
DOI: 10.1002/chemv.201000047 - In Chemistry of a Hangover — Alcohol and its Consequences
Klaus Roth asks how can a tiny molecule like ethanol be at the root of so much human misery?
DOI: 10.1002/chemv.201000074




