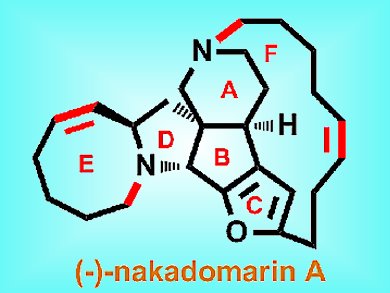
(–)-Nakadomarin A is an alkaloid that was first isolated from a marine sponge in 1997. It has a unique 8/5/5/5/15/6 acyclic full-ring structure with four stereogenic centers and an embedded furan. It has numerous interesting biological properties, including cytotoxicity against murine lymphoma cells and antimicrobial activity. Many total and formal syntheses have been previously reported for this molecule, but Hongbin Zhai and co-workers, Shanghai Institute of Organic Chemistry, Shanghai, describe a more practical and efficient route.
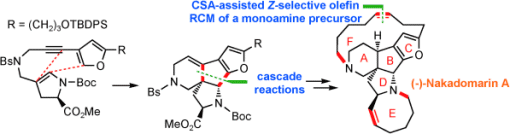
In their method, the 15-sided F ring was formed by a practical (–)-camphorsulfonic acid-assisted Z-selective olefin ring-closing metathesis of the less polar monoamine substrate, rather than the more polar diamine substrate used in previous syntheses. The tetracyclic ABCD core was assembled through a novel and efficient cascade reaction sequence promoted by PtCl2.
Images: © Wiley-VCH
- Efficient Total Synthesis of Marine Alkaloid (–)-Nakadomarin A
B. Cheng, F. Wu, X. Yang, Y. Zhou, X. Wan, H. Zhai,
Chem. Eur. J. 2011.
DOI: 10.1002/chem.201102101